Advertisements
Online Mock Tests
Chapters
2: Introduction to Analytical Chemistry
3: Basic Analytical Techniques
4: Structure of Atom
5: Chemical Bonding
6: Redox Reactions
7: Modern Periodic Table
8: Elements of Group 1 and 2
9: Elements of Group 13, 14 and 15
▶ 10: States of Matter
11: Adsorption and Colloids
12: Chemical Equilibrium
13: Nuclear Chemistry and Radioactivity
14: Basic Principles of Organic Chemistry
15: Hydrocarbons
16: Chemistry in Everyday Life
![Balbharati solutions for Chemistry [English] 11 Standard chapter 10 - States of Matter Balbharati solutions for Chemistry [English] 11 Standard chapter 10 - States of Matter - Shaalaa.com](/images/chemistry-english-11-standard_6:1ddd95908cb04440a83d42566c3337de.jpg)
Advertisements
Solutions for Chapter 10: States of Matter
Below listed, you can find solutions for Chapter 10 of Maharashtra State Board Balbharati for Chemistry [English] 11 Standard.
Balbharati solutions for Chemistry [English] 11 Standard 10 States of Matter Exercises [Pages 157 - 159]
Select and write the most appropriate alternatives from the given choices.
The unit of viscosity is
dynes
newton
gram
poise
Select and write the most appropriate alternatives from the given choices.
Which of the following is true for 2 moles of an ideal gas?
PV = nRT
PV = RT
PV = 2RT
PV = T
Select and write the most appropriate alternatives from the given choices.
Intermolecular forces in liquid are -
greater than gases
less than solids
greater than gases and less than solids
greater than solids
Select and write the most appropriate alternatives from the given choices.
Interactive forces are __________ in an ideal gas.
nil
small
large
same as that of real gases
Select and write the most appropriate alternatives from the given choices.
At constant temperature the pressure of 22.4 dm3 volume of an ideal gas was increased from 105 kPa to 210 kPa, New volume could be -
44.8 dm3
11.2 dm3
22.4 dm3
5.6 dm3
Answer in one sentence.
Name the term used for the mixing of different gases by random molecular motion and frequent collision.
Answer in one sentence.
The pressure that each individual gas would exert if it were alone in the container, what do we call it as?
Answer in one sentence.
When a gas is heated the particles move more quickly. What is the change in the volume of a heated gas if the pressure is kept constant?
Answer in one sentence.
A bubble of methane gas rises from the bottom of the North sea. What will happen to the size of the bubble as it rises to the surface?
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert the following temperature from degree Celcius to kelvin.
25° C
Convert the following temperature from degree Celcius to kelvin.
−197° C
Convert the following temperature from degree Celcius to kelvin.
273° C
Convert the following pressure value into Pascals.
10 atmosphere
Convert the following pressure value into Pascals.
1 kPa
Convert the following pressure value into Pascals.
107000 Nm−2
Convert the following pressure value into Pascals.
1 atmosphere
Convert exactly 1.5 atm to pascals
Convert 89 kPa to newton per square metre (Nm−2)
Convert 101.325 kPa to bar.
Convert −100° C to kelvin
Convert 0.124 torr to the standard atmosphere
If the density of a gas is measured at constant temperature and pressure then which of the following statement is correct?
Density is directly proportional to molar mass of the gas.
Greater the density greater is the molar mass of the gas.
If density, temperature and pressure is given ideal gas equation can be used to find molar mass.
All the above statements are correct.
Observe the following conversion.
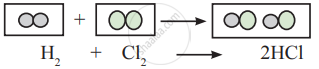
Is the above reaction is in accordance with the principle of stoichiometry?
Observe the following conversion.
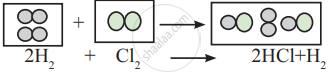
Is the above reaction is in accordance with the principle of stoichiometry?
Hot air balloons float in the air because of the low density of the air inside the balloon. Explain this with the help of an appropriate gas law.

Identify the gas laws from the following diagram.
| Diagram | Gas laws |
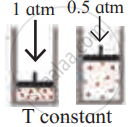 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
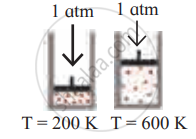 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
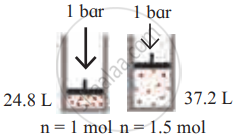 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
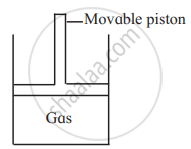
Show diagrammatically the changes in the position of the piston, if pressure is increased from 1.0 bar to 2.0 bar at a constant temperature.
Consider a sample of a gas in a cylinder with a movable piston.

Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 300 K to 150 K at constant pressure.
Consider a sample of a gas in a cylinder with a movable piston.

Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 400 K to 300 K, and pressure is decreased from 4 bar to 3 bar.
List the characteristic physical properties of the gases.
Define the term polarizability
Define the term Hydrogen bond
Define the term Aqueous tension
Define the term Dipole moment
Would it be easier to drink water with a straw on the top of Mount Everest or at the base? Explain.
Identify type of the intermolecular forces in the following compound.
CH3-OH
Identify type of the intermolecular forces in the following compound.
CH2=CH2
Identify type of the intermolecular forces in the following compound.
CHCl3
Identify type of the intermolecular forces in the following compound.
CH2Cl2
Name the types of intermolecular forces present in Ar.
Name the types of intermolecular forces present in Cl2.
Name the types of intermolecular forces present in CCl4.
Name the types of intermolecular forces present in HNO3.
Match the pairs of the following:
| Column ‘A’ | Column ‘B’ |
| a. Boyle’s law | i. at constant pressure and volume |
| b. Charles’ law | ii. at constant temperature |
| iii. at constant pressure |
Write the statement for Boyle’s law
Write the statement for Charles’ law
Differentiate between Real gas and Ideal gas.
State and write the mathematical expression for Dalton’s law of partial pressure and explain it with a suitable example.
Derive an Ideal gas equation. Mention the terms involved in it.
Write how ideal gas equation is utilised to obtain combined gas law.
With the help of the graph answer the following -

At constant temperature, the Graph shows the relationship between pressure and volume. Represent the relation mathematically.
With the help of the graph answer the following -

At constant temperature, Identify the law.
With the help of the graph answer the following -

At constant temperature, Write the statement of law.
Write Postulates of the kinetic theory of gases.
Write a short note on Vapour pressure.
Write a short note on Surface tension.
Write a short note on Viscosity.
Solve the following.
A balloon is inflated with helium gas at room temperature of 25°C and at 1 bar pressure when its initial volume is 2.27L and allowed to rise in the air. As it rises in the air external pressure decreases and the volume of the gas increases till finally, it bursts when external pressure is 0.3bar. What is the limit at which the volume of the balloon can stay inflated?
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is increased by 10°C.
Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is decreased by 10°C.
Solve the following.
A hot air balloon has a volume of 2800 m3 at 99°C. What is the volume if the air cools to 80°C?

Solve the following.
At 0°C, a gas occupies 22.4 liters. How much hot must be the gas in celsius and in kelvin to reach a volume of 25.0 liters?
Solve the following.
A 20 L container holds 0.650 mol of He gas at 37°C at a pressure of 628.3 bar. What will be new pressure inside the container if the volume is reduced to 12 L. The temperature is increased to 177°C and 1.25 mol of additional He gas was added to it?
Solve the following.
Nitrogen gas is filled in a container of volume 2.32 L at 32°C and 4.7 atm pressure. Calculate the number of moles of the gas.
Solve the following.
At 25°C and 760 mm of Hg pressure, a gas occupies 600 mL volume. What will be its pressure at the height where the temperature is 10°C and the volume of the gas 640 mL?
Solve the following.
A neon-dioxygen mixture contains 70.6 g dioxygen and 167.5g neon. If the pressure of the mixture of the gases in the cylinder is 25 bar. What is the partial pressure of dioxygen and neon in the mixture?
Solve the following.
Calculate the pressure in atm of 1.0 mole of helium in a 2.0 dm3 container at 20.0°C.
Solve the following.
Calculate the volume of 1 mole of a gas at exactly 20°C at a pressure of 101.35 kPa.
Solve the following.
Calculate the number of molecules of methane in 0.50 m3 of the gas at a pressure of 2.0 × 102 kPa and a temperature of exactly 300 K.
Solutions for 10: States of Matter
![Balbharati solutions for Chemistry [English] 11 Standard chapter 10 - States of Matter Balbharati solutions for Chemistry [English] 11 Standard chapter 10 - States of Matter - Shaalaa.com](/images/chemistry-english-11-standard_6:1ddd95908cb04440a83d42566c3337de.jpg)
Balbharati solutions for Chemistry [English] 11 Standard chapter 10 - States of Matter
Shaalaa.com has the Maharashtra State Board Mathematics Chemistry [English] 11 Standard Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Balbharati solutions for Mathematics Chemistry [English] 11 Standard Maharashtra State Board 10 (States of Matter) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Balbharati textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] 11 Standard chapter 10 States of Matter are Introduction of States of Matter: Gaseous and Liquid States, Intermolecular Forces, Characteristic Properties of Gases, The Gas Laws, Ideal Gas Equation, Kinetic Molecular Theory of Gases, Deviation from Ideal Behaviour, Liquefaction of Gases and Critical Constant, Liquid State.
Using Balbharati Chemistry [English] 11 Standard solutions States of Matter exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Balbharati Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Chemistry [English] 11 Standard students prefer Balbharati Textbook Solutions to score more in exams.
Get the free view of Chapter 10, States of Matter Chemistry [English] 11 Standard additional questions for Mathematics Chemistry [English] 11 Standard Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.
