Advertisements
Advertisements
Question
Identify type of the intermolecular forces in the following compound.
CHCl3
Solution
Dipole-dipole interactions and London dispersion forces
APPEARS IN
RELATED QUESTIONS
Select and write the most appropriate alternatives from the given choices.
Intermolecular forces in liquid are -
Observe the following conversion.
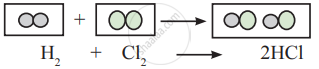
Is the above reaction is in accordance with the principle of stoichiometry?
Observe the following conversion.
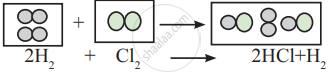
Is the above reaction is in accordance with the principle of stoichiometry?
Define the term polarizability
Define the term Hydrogen bond
Define the term Aqueous tension
Identify type of the intermolecular forces in the following compound.
CH3-OH
Identify type of the intermolecular forces in the following compound.
CH2=CH2
Identify type of the intermolecular forces in the following compound.
CH2Cl2
Name the types of intermolecular forces present in Ar.
Name the types of intermolecular forces present in Cl2.
The force of attraction between any two nonpolar molecules is ____________.
Based on intermolecular forces, which of the following is a fibre?
Which of the following is a hydrogen bonded molecular solid?
Which of the following molecules have London forces as predominant intermolecular force of attraction?
In ____________, hydrogen bonding is stronger than corresponding alcohols.
What type of inter molecular force is present between magnesium chloride and water?
In which of the following compound intra molecular hydrogen bonding is present?
If 'Q' is the magnitude of charge and 'r' is the distance between the centres of positive and negative charges then dipole moment (µ) is given by ______.
Identify the type of intermolecular force present between benzene and ammonia.
