Advertisements
Advertisements
Question
Convert 101.325 kPa to bar.
Solution
101.325 kPa to bar:
1 bar = 1.0 × 105 Pa
= 1.0 × 102 kPa
∴ 100 kPa = 1 bar
∴ 101.325 kPa = `(1xx101.325)/100`
= 1.01325 bar
APPEARS IN
RELATED QUESTIONS
State (i) the three variables for gas laws and (ii) SI units of these variables.
Give reason for the following:
Gases have a lower density compared to solids or liquids.
Convert the following pressure value into Pascals.
10 atmosphere
Hot air balloons float in the air because of the low density of the air inside the balloon. Explain this with the help of an appropriate gas law.

Identify the gas laws from the following diagram.
| Diagram | Gas laws |
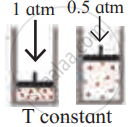 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
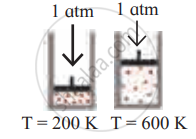 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
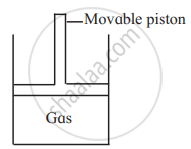
Show diagrammatically the changes in the position of the piston, if pressure is increased from 1.0 bar to 2.0 bar at a constant temperature.
With the help of the graph answer the following -

At constant temperature, the Graph shows the relationship between pressure and volume. Represent the relation mathematically.
With the help of the graph answer the following -

At constant temperature, Write the statement of law.
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is increased by 10°C.
Solve the following.
A hot air balloon has a volume of 2800 m3 at 99°C. What is the volume if the air cools to 80°C?

Solve the following.
At 0°C, a gas occupies 22.4 liters. How much hot must be the gas in celsius and in kelvin to reach a volume of 25.0 liters?
Use of hot air balloon in sports and meteorological observation is an application of
State Boyle's law.
Explain the following observation.
Liquid ammonia bottle is cooled before opening the seal
Explain the following observation.
The type of an automobile is inflated to slightly lesser pressure in summer than in winter
Of two samples of nitrogen gas, sample A contains 1.5 moles of nitrogen in a vessel of the volume of 37.6 dm3 at 298 K, and sample B is in a vessel of volume 16.5 dm3 at 298 K. Calculate the number of moles in sample B.
A small bubble rises from the bottom of a lake where the temperature and pressure are 6°C and 4 atm. to the water surface, where the temperature is 25°C and pressure is 1 atm. Calculate the final volume in (mL) of the bubble, if its initial volume is 1.5 mL.
Hydrochloric acid is treated with a metal to produce hydrogen gas. Suppose a student carries out this reaction and collects a volume of 154.4 × 10−3 dm3 of a gas at a pressure of 742 mm of Hg at a temperature of 298 K. What mass of hydrogen gas (in mg) did the student collect?
At 25°C and 1 atm, a cylinder containing 10 L of an ideal gas is connected to the empty cylinder with a capacity of 20 L. The pressures exerted by gas m both the cylinders will be ____________.
A certain sample of gas has a volume of 0.2 L at one atmosphere pressure and 273.15 K. What is the volume of gas at 273.15°C at same pressure?
Volume of a balloon at 25°C and 1 bar pressure is 2.27 L. If the pressure of the gas in balloon is reduced to 0.227 bar, what is the rise in volume of a gas?
The volume of 400 cm3 chlorine gas at 400 mm of Hg is decreased to 200 cm3 at constant temperature. What is the new pressure of gas?
If 300 mL of a gas at 26.85°C is cooled to 6.85°C at constant pressure. What will be the final volume of gas?
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
