Advertisements
Advertisements
Question
Hydrochloric acid is treated with a metal to produce hydrogen gas. Suppose a student carries out this reaction and collects a volume of 154.4 × 10−3 dm3 of a gas at a pressure of 742 mm of Hg at a temperature of 298 K. What mass of hydrogen gas (in mg) did the student collect?
Solution
Given, V = 154.4 × 10−3 dm3
P = 742 mm of Hg
T = 298 K
m = ?
n = `"PV"/"RT"`
= `(742 "mm Hg" xx 154.4 xx 10^-13 "L")/(62 "mm Hg L K"^-1 "mol"^-1 xx 298 "K")`
= 0.006 mol
n = `"Mass"/("Molar mass")`
Mass = n × Molar Mass
= 0.0006 × 2.016
= 0.0121 g
= 12.1 mg
APPEARS IN
RELATED QUESTIONS
Convert the following temperature from degree Celcius to kelvin.
25° C
Hot air balloons float in the air because of the low density of the air inside the balloon. Explain this with the help of an appropriate gas law.

Identify the gas laws from the following diagram.
| Diagram | Gas laws |
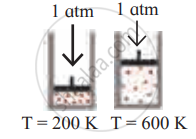 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
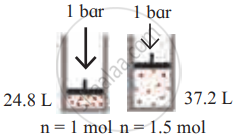 |
______________ |
Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is increased by 10°C.
Solve the following.
A hot air balloon has a volume of 2800 m3 at 99°C. What is the volume if the air cools to 80°C?

Name two items that can serve as a model for Gay Lusaac’s law and explain.
Sulphur hexafluoride is a colourless, odourless gas; calculate the pressure exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 dm3 at 69.5 °C, assuming ideal gas behaviour
A certain mass of a gas occupies a volume of 2 dm3 at STP. At what temperature the volume of gas becomes double, keeping the pressure constant?
10 g of gas at one atomospheric pressure is cooled from 273.15°C to 0°C keeping the volume constant. What is the final pressure?
