Advertisements
Advertisements
Question
Use of hot air balloon in sports and meteorological observation is an application of
Options
Boyle’s law
Newton’s law
Kelvin’s law
Brown’s law
Solution
Boyle’s law
APPEARS IN
RELATED QUESTIONS
What would be the mass of CO2 occupying a volume of 44 litres at 25°C and 750 mm pressure.
State the following:
The absolute temperature of a gas at 7°C
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
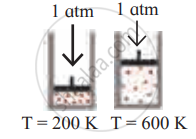 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
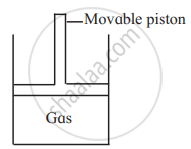
Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 400 K to 300 K, and pressure is decreased from 4 bar to 3 bar.
Solve the following.
A balloon is inflated with helium gas at room temperature of 25°C and at 1 bar pressure when its initial volume is 2.27L and allowed to rise in the air. As it rises in the air external pressure decreases and the volume of the gas increases till finally, it bursts when external pressure is 0.3bar. What is the limit at which the volume of the balloon can stay inflated?
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

A sample of gas at 15°C at 1 atm. has a volume of 2.58 dm3. When the temperature is raised to 38°C at 1 atm does the volume of the gas Increase? If so, calculate the final volume.
At 25°C and 1 atm, a cylinder containing 10 L of an ideal gas is connected to the empty cylinder with a capacity of 20 L. The pressures exerted by gas m both the cylinders will be ____________.
If 300 mL of a gas at 26.85°C is cooled to 6.85°C at constant pressure. What will be the final volume of gas?
At what temperature, the volume of gas would become zero?
