Advertisements
Advertisements
प्रश्न
Use of hot air balloon in sports and meteorological observation is an application of
पर्याय
Boyle’s law
Newton’s law
Kelvin’s law
Brown’s law
उत्तर
Boyle’s law
APPEARS IN
संबंधित प्रश्न
What would be the mass of CO2 occupying a volume of 44 litres at 25°C and 750 mm pressure.
Convert the following pressure value into Pascals.
10 atmosphere
Convert the following pressure value into Pascals.
107000 Nm−2
Convert 89 kPa to newton per square metre (Nm−2)
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
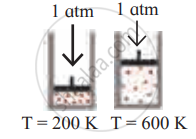 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
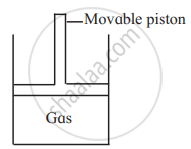
Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 400 K to 300 K, and pressure is decreased from 4 bar to 3 bar.
Match the pairs of the following:
| Column ‘A’ | Column ‘B’ |
| a. Boyle’s law | i. at constant pressure and volume |
| b. Charles’ law | ii. at constant temperature |
| iii. at constant pressure |
According to Andrews isothermals at what temperature the carbon dioxide gas starts to condense at 73 atmosphere?
At what temperature, the volume of gas would become zero?
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
