Advertisements
Advertisements
Question
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
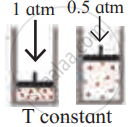 |
______________ |
Solution
| Diagram | Gas laws |
 |
Boyle’s law |
APPEARS IN
RELATED QUESTIONS
What would be the mass of CO2 occupying a volume of 44 litres at 25°C and 750 mm pressure.
Give reason for the following:
Gases exert pressure in all directions.
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert the following temperature from degree Celcius to kelvin.
273° C
Convert the following pressure value into Pascals.
107000 Nm−2
Convert −100° C to kelvin
Convert 0.124 torr to the standard atmosphere
Consider a sample of a gas in a cylinder with a movable piston.
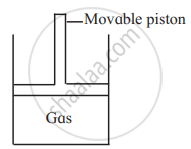
Show diagrammatically the changes in the position of the piston, if pressure is increased from 1.0 bar to 2.0 bar at a constant temperature.
Consider a sample of a gas in a cylinder with a movable piston.

Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 300 K to 150 K at constant pressure.
Write the statement for Boyle’s law
Write the statement for Charles’ law
With the help of the graph answer the following -

At constant temperature, the Graph shows the relationship between pressure and volume. Represent the relation mathematically.
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is increased by 10°C.
The temperatures at which real gases obey the ideal gas laws over a wide range of pressure is called __________.
Explain the following observation.
Aerated water bottles are kept under water during summer
A sample of gas at 15°C at 1 atm. has a volume of 2.58 dm3. When the temperature is raised to 38°C at 1 atm does the volume of the gas Increase? If so, calculate the final volume.
Of two samples of nitrogen gas, sample A contains 1.5 moles of nitrogen in a vessel of the volume of 37.6 dm3 at 298 K, and sample B is in a vessel of volume 16.5 dm3 at 298 K. Calculate the number of moles in sample B.
A small bubble rises from the bottom of a lake where the temperature and pressure are 6°C and 4 atm. to the water surface, where the temperature is 25°C and pressure is 1 atm. Calculate the final volume in (mL) of the bubble, if its initial volume is 1.5 mL.
For a given mass of an ideal gas, which of the following statements is CORRECT?
At what temperature the volume of a gas becomes absolutely zero?
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
