Advertisements
Advertisements
Questions
Give reason for the following:
Gases exert pressure in all directions.
Gases unlike solids and liquids exert pressure in all directions.
Solution 1
The gas particles have a very weak attractive force between them and move randomly ultimately exert pressure in all direction. Their molecules are bouncing all around the container because they are so small, then gravity has very little effect on them.
Solution 2
Impact of gas molecules with high velocity causes pressure to be exerted on the walls.
RELATED QUESTIONS
Convert the following temperature from degree Celcius to kelvin.
−197° C
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
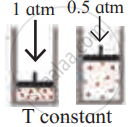 |
______________ |
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
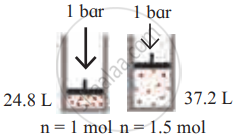 |
______________ |
Write the statement for Charles’ law
With the help of the graph answer the following -

At constant temperature, Identify the law.
Give the mathematical expression that relates gas volume and moles.
Explain the following observation.
Aerated water bottles are kept under water during summer
Explain the following observation.
Liquid ammonia bottle is cooled before opening the seal
A sample of gas has a volume of 8.5 dm3 at an unknown temperature. When the sample is submerged in ice water at 0°C, its volume gets reduced to 6.37 dm3. What is its initial temperature?
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
