Advertisements
Advertisements
Question
With the help of the graph answer the following -

At constant temperature, Identify the law.
Solution
The graph represents Boyle’s law as it gives a relation between pressure and volume at a constant temperature.
APPEARS IN
RELATED QUESTIONS
What would be the mass of CO2 occupying a volume of 44 litres at 25°C and 750 mm pressure.
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
Answer in one sentence.
A bubble of methane gas rises from the bottom of the North sea. What will happen to the size of the bubble as it rises to the surface?
Convert the following temperature from degree Celcius to kelvin.
−197° C
Convert the following pressure value into Pascals.
10 atmosphere
Convert 0.124 torr to the standard atmosphere
Hot air balloons float in the air because of the low density of the air inside the balloon. Explain this with the help of an appropriate gas law.

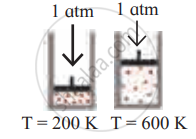
Identify the gas laws from the following diagram.
| Diagram | Gas laws |
 |
______________ |
Consider a sample of a gas in a cylinder with a movable piston.
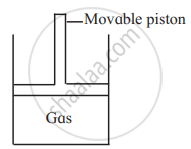
Show diagrammatically the changes in the position of the piston, if the temperature is decreased from 300 K to 150 K at constant pressure.
Match the pairs of the following:
| Column ‘A’ | Column ‘B’ |
| a. Boyle’s law | i. at constant pressure and volume |
| b. Charles’ law | ii. at constant temperature |
| iii. at constant pressure |
Write the statement for Charles’ law
Solve the following.
A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be the final volume to change the pressure to 3.5 atm?

Solve the following.
A hot air balloon has a volume of 2800 m3 at 99°C. What is the volume if the air cools to 80°C?

Use of hot air balloon in sports and meteorological observation is an application of
State Boyle's law.
Name two items that can serve as a model for Gay Lusaac’s law and explain.
Explain the following observation.
Aerated water bottles are kept under water during summer
Explain the following observation.
The type of an automobile is inflated to slightly lesser pressure in summer than in winter
A sample of gas at 15°C at 1 atm. has a volume of 2.58 dm3. When the temperature is raised to 38°C at 1 atm does the volume of the gas Increase? If so, calculate the final volume.
A sample of gas has a volume of 8.5 dm3 at an unknown temperature. When the sample is submerged in ice water at 0°C, its volume gets reduced to 6.37 dm3. What is its initial temperature?
Sulphur hexafluoride is a colourless, odourless gas; calculate the pressure exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 dm3 at 69.5 °C, assuming ideal gas behaviour
For a given mass of an ideal gas, which of the following statements is CORRECT?
According to Andrews isothermals, the minimum temperature at which carbon dioxide gas obeys Boyles law is ______.
Volume of a balloon at 25°C and 1 bar pressure is 2.27 L. If the pressure of the gas in balloon is reduced to 0.227 bar, what is the rise in volume of a gas?
Isochor is the graph plotted between ______.
If 300 mL of a gas at 26.85°C is cooled to 6.85°C at constant pressure. What will be the final volume of gas?
At what temperature, the volume of gas would become zero?
