Advertisements
Advertisements
Question
The temperatures at which real gases obey the ideal gas laws over a wide range of pressure is called __________.
Options
Critical temperature
Boyle temperature
Inversion temperature
Reduced temperature
Solution
The temperatures at which real gases obey the ideal gas laws over a wide range of pressure is called Boyle temperature.
APPEARS IN
RELATED QUESTIONS
Convert the following temperature from degree Celcius to kelvin.
−15° C
Convert the following pressure value into Pascals.
10 atmosphere
Hot air balloons float in the air because of the low density of the air inside the balloon. Explain this with the help of an appropriate gas law.

Identify the gas laws from the following diagram.
| Diagram | Gas laws |
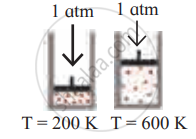 |
______________ |
Write the statement for Charles’ law
Solve the following.
The volume of a given mass of a gas at 0°C is 2 dm3. Calculate the new volume of the gas at constant pressure when the temperature is decreased by 10°C.
Solve the following.
At 0°C, a gas occupies 22.4 liters. How much hot must be the gas in celsius and in kelvin to reach a volume of 25.0 liters?
Give the mathematical expression that relates gas volume and moles.
A certain sample of gas has a volume of 0.2 L at one atmosphere pressure and 273.15 K. What is the volume of gas at 273.15°C at same pressure?
The number of molecules in 8.96 litres of gas at 0°C and 1 atm. pressure is approximately ______.
