Advertisements
Advertisements
Question
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds which are functional group isomers.
Solution
The compounds with the same molecular formula but different functional groups are said to be functional group isomers.
Alcohols are found to be the functional isomers of ether. In the given structures, I, II, III, IV alcohol functional group is present and V, VI, VII contains ether functional group. Hence, I and V, I and VI, I and VII, II and V, II and VI, II and VII, III and V, III and VI etc are functional group isomers.
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
2-Ethylbut-1-ene
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Find out the type of isomerism exhibited by the following pair.

What type(s) of isomerism is(are) shown by [Co(NH3)4Br2]Cl?
The type of isomerism possible in 2-butene is ____________.
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}\ce{^+OH}\\||\\
\ce{H - C - OH}\end{array}\]
\[\begin{array}{cc}\ce{OH}\phantom{.}\\|\phantom{...}\\
\ce{H - C^+ - OH}\end{array}\]
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents position isomerism.
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents chain isomerism.
Compounds with same molecular formula but differing in their structures are said to be structural isomers. What type of structural isomerism is shown by
CH3 – S – CH2 – CH2 – CH3
And
\[\begin{array}{cc}
\phantom{.....................}\ce{CH3}\\
\phantom{................}/\\
\phantom{}\ce{CH3 - S - CH}\\
\phantom{...............}\backslash\\
\phantom{....................}\ce{CH3}
\end{array}\]
Assertion (A): Pent- 1- ene and pent- 2- ene are position isomers.
Reason (R): Position isomers differ in the position of functional group or a substituent.
Assertion (A): The compound cyclooctane has the following structural formula: ![]()
It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R): (4n + 2)π electrons rule does not hold good and ring is not planar.
Tautomerism is exhibited by ______.
Which of the following does NOT exhibit geometrical isomerism?
The correct stereochemical name of
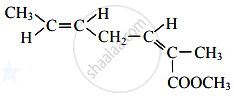
How many structural isomers possible of the molecular formula C3H6O (excluding enol form)?
Which of the following pairs of compounds are positional isomers?
Which of the following reactions will not produce a racemic product?
Which of the following pairs of compounds is an example of position isomerism?
