Advertisements
Advertisements
Question
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
3,4-Dimethyl-hept-3-ene
Solution
3, 4-Dimethylhept-3-ene undergoes ozonolysis as:
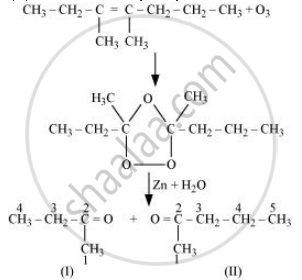
The IUPAC name of Product (I) is butan-2-one and Product (II) is Pentan-2-one.
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
2-Ethylbut-1-ene
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{H}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{D}
\end{array}\]
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{D}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{H}\end{array}\]
Find out the type of isomerism exhibited by the following pair.
CH3 – CH2 – NH – CH2 - CH3 and CH3 - NH - CH2 - CH2 - CH3
Find out the type of isomerism exhibited by the following pair.
\[\begin{array}{cc}
\ce{CH3 - CH - CH2 - CH3 and CH3 - CH2 - O - CH2 - CH3}\\|\phantom{...........................................}\\
\ce{OH}\phantom{.........................................}\end{array}\]
Find out the type of isomerism exhibited by the following pair.

Molecular formula of the functional isomer of methyl formate is ____________.
What type(s) of isomerism is(are) shown by [Co(NH3)4Br2]Cl?
But-1-ene and But-2-ene are examples of ____________.
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}\ce{^+OH}\\||\\
\ce{H - C - OH}\end{array}\]
\[\begin{array}{cc}\ce{OH}\phantom{.}\\|\phantom{...}\\
\ce{H - C^+ - OH}\end{array}\]
In which of the following, functional group isomerism is not possible?
Which of the following pairs are not functional group isomers?
| I. | \[\begin{array}{cc} \phantom{.......................}\ce{O}\\ \phantom{.......................}||\\ \ce{CH3 - CH2 - CH2 - CH2 - C - H} \end{array}\] |
| II. | \[\begin{array}{cc} \phantom{.................}\ce{O}\\ \phantom{.................}||\\ \ce{CH3 - CH2 - CH2 - C - H} \end{array}\] |
| III. | \[\begin{array}{cc} \ce{CH3 - CH2 - C - CH2 - CH3}\\ \phantom{}||\\ \phantom{}\ce{O} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - C - H}\\ \phantom{...}|\phantom{............}||\phantom{}\\ \phantom{...}\ce{CH3}\phantom{.........}\ce{O}\phantom{} \end{array}\] |
(i) II and III
(ii) II and IV
(iii) I and IV
(iv) I and II
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents position isomerism.
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents chain isomerism.
The molecules having dipole moment are:
(i) 2,2-Dimethylpropane
(ii) trans-Pent-2-ene
(iii) cis-Hex-3-ene
(iv) 2, 2, 3, 3 - Tetramethylbutane.
Assertion (A): The compound cyclooctane has the following structural formula: ![]()
It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R): (4n + 2)π electrons rule does not hold good and ring is not planar.
Tautomerism is exhibited by ______.
Which of the following does NOT exhibit geometrical isomerism?
Ether and alcohol are ______.
Which type of isomerism can not be shown by benzaldoxime?
Acetamide is isomer of ______.
Which one of the following pairs are called position isomers?
The number of acyclic structural isomers (including geometrical isomers) for pentene are ______.
Which of the following pairs of compounds are positional isomers?
The number of geometrical isomers from [Co(NH3)3(NO2)3] is ______.
