Advertisements
Advertisements
Question
How will you prepare the following from nitric acid?
Sodium nitrate
Solution
Sodium nitrate can be prepared by the action of dil. \[\ce{HNO3}\] on sodium bicarbonate or sodium carbonate.
\[\ce{\underset{Sodium bicarbonate}{NaHCO3} + HNO3_{(dil)} -> \underset{Sodium nitrate}{NaNO3} + CO2 + H2O}\]
\[\ce{\underset{Sodium carbonate}{Na2CO3} + 2HNO3_{(dil)} -> \underset{Sodium nitrate}{2NaNO3} + CO2 + H2O}\]
APPEARS IN
RELATED QUESTIONS
How will you prepare the following from nitric acid?
Magnesium nitrate
Name a metal nitrate which on heating is changed into metal.
Explain with the help of a balanced equation, the brown ring test for nitric acid.
Name the Following:
Product obtained by heating concentrated nitric acid.
Describe the Ostwald's process for the manufacture of nitric acid with labeled diagram.
Nitric acid cannot be concentrated beyond 68% by the distillation of a dilute solution of HNO3, State the reason.
Explain with the help of a balanced equation, the brown ring test for nitric acid.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: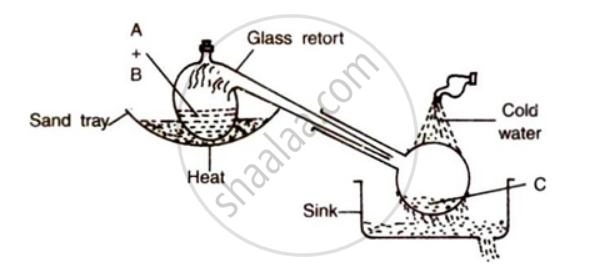
(i) Name A (a liquid), B( a Solid) and C(a liquid).
(ii) write the balanced chemical equation to show how nitric acid undergoes decomposition.
(iii) Write the balanced chemical equation for the reaction in which copper is oxidized by concentrated nitric acid.
X, Y, and Z are three crystalline solids that are soluble in water and have common anion.
To help you to identify X, Y and Z, you are provided with the following experimental observations. Copy and complete the corresponding inferences in (a) to (e).
- A reddish-brown gas is obtained when X, Y, and Z are separately warmed with concentrated sulphuric acid and copper turning added to the mixture.
INFERENCE 1: The common anion is the ______ ion. - When X is heated, it melts and gives off only one gas which re-lights a glowing splint.
INFERENCE 2: The cation in X is either ______ or ______. - The action of heat on Y produces a reddish-brown gas and yellow residue which fuses with a glass of the test tube.
INFERENCE 3: The metal ion present in Y is the ______ ion. - When Z is heated, it leaves no residue. Warming Z with sodium hydroxide solution liberates a gas which turns moist red litmus paper blue.
INFERENCE 4: Z contains the ______ cation. - Write the equations for the following reactions:
- X and concentrated sulphuric acid (below 200°C). (One equation only for either of the cations given in INFERENCE 2)
- The action of heat on Y.
- Concentrated nitric acid is added to copper turnings kept in a beaker.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
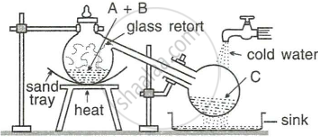
- Name A (a liquid), B (a solid), and C (a liquid). (Do not give the formulae).
- Write an equation to show how nitric acid undergoes decomposition.
- Write the equation for the reaction in which copper is oxidised by concentrated nitric acid.
