Advertisements
Advertisements
Question
Ice-cream at 0°C feels colder than water at 0°C. Give reason for this observation.
Solution
We know that we need to supply about 340 joule of heat per g, to convert ice at 0°C, into water at 0°C. It follows that that ice-cream, at 0°C, will draw more heat (about 340 joule more per gram) from burbody than water at 0°C. It, therefore, feels colder than water at 0°C.
APPEARS IN
RELATED QUESTIONS
Why do bottled soft drinks get cooled, more quickly by the ice cubes than by the iced water, both at 0℃?
Name the radiations for which the green house gases are transparent ?
State three ways to minimize the global warming.
The specific heat capacity of a body depends on _____________ .
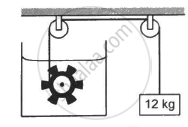
Figure shows a paddle wheel coupled to a mass of 12 kg through fixed frictionless pulleys. The paddle is immersed in a liquid of heat capacity 4200 J K−1 kept in an adiabatic container. Consider a time interval in which the 12 kg block falls slowly through 70 cm. (a) How much heat is given to the liquid? (b) How much work is done on the liquid? (c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle.
(i) State whether the specific heat capacity of a substance remains the same when its state changes from solid to liquid.
(ii) Give one example to support your answer.
Will the value of specific heat’capacity and specific latent heat of a substance change if the scale is °F instead of °C?
Read this activity and answer the following questions.
- Take three spheres of iron, copper and lead. the lead of equal mass.
- Put all the three spheres in boiling water in the beaker for some time.
- Take the three spheres out of the water.
- All the spheres will be at a temperature 100 °C.
- Put them immediately on the thick slab of wax.
- Note, the depth that each of the sphere goes into the wax.
Questions:
- Which property is determined from this activity?
- Give name to that property.
- Explain the term principal of heat exchange with the help of this activity.
