Advertisements
Advertisements
Question
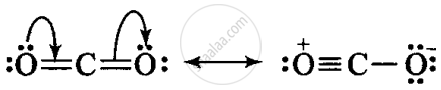
Identify the correct resonance structures of carbon dioxide from the ones given below:
(i) O – C ≡ O
(ii) O = C = O
(iii) –O ≡ C – O+
(iv) –O – C ≡ O+
Solution
(ii) O = C = O
(iv) –O – C ≡ O+
Explanation:
The following is the resonance structure of CO2.
APPEARS IN
RELATED QUESTIONS
Write the resonance structure of `"CO"_3^(2-)`.
What is the state of hybridisation of carbon in `"CO"_3^(2-)`.
What is the state of hybridisation of carbon in Diamond ?
What is the state of hybridisation of carbon in graphite?
How is an excessive content of CO2 responsible for global warming?
Give one method for industrial preparation and one for laboratory preparation of CO2.
Give one method for industrial preparation and one for laboratory preparation of CO.
Which of the following can be directly solidified from the gaseous state?
What happens when steam is passed over red-hot carbon?
Water does not produce CO on reacting with ______.
