Advertisements
Advertisements
Question
In the following table, the positions of six elements A, B, C, D, E and F are given as they are in the Modern Periodic Table :
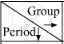 |
1 | 2 | 3-12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 2 | A | B | C | D | |||||
| 3 | E | F |
On the basis of the above table, answer the following questions :
(i) Name the element which forms only covalent compounds.
(ii) Name the element which is a metal with valency three.
(iii) Name the element which is a non-metal with valency three.
(iv) Out of B and C, whose atomic radius is bigger and why ?
(v) Write the common name for the family to which the elements D and F belong.
Solution
(i) Element E is present in Group 14. It has a valency of four. Thus, among the given elements, it forms only covalent compounds.
(ii) Element B is present in Group 13. Thus, among the given elements, it is a metal with valency three.
(iii) Element C is present in Group 15. It is a non-metal with five valence electrons. Thus, it has a valency of three.
(iv) Elements B and C are present in period 2 of the periodic table. On going from left to right in the periodic table, the atomic radii of elements decrease due to increase in effective nuclear charge that pulls the valence electrons close to the nucleus. Thus, out of B and C, the atomic radius of B is bigger.
(v) Elements D and F are present in Group 18 of the periodic table. They belong to the family of inert/noble gases.
APPEARS IN
RELATED QUESTIONS
Two elements ‘A’ and ‘B’ belong to the 3rd period of Modern periodic table and are in group 2 and 13, respectively. Compare their following characteristics in tabular form:-
(a) Number of electrons in their atoms
(b) Size of their atoms
(c) Their tendencies to lose electrons
(d) The formula of their oxides
(e) Their metallic character
(f) The formula of their chlorides
Differentiate between Normal elements and Transition elements.
Name the non-metals present in period 2 and metals in period 3.
name the elements in period 1.
An element 'X' (Atomic number – 20) burns in the presence of oxygen to form a basic oxide.
(a) Identify the element and write its electronic configuration.
(b) State its group number and period number in the Modern Periodic Table.
(c) Write a balanced chemical equation for the reaction when this oxide is dissolved in water.
Four elements P, Q, R and S belong to the third period of the Modern Periodic Table and have respectively 1, 3, 5 and 7 electrons in their outermost shells. Write the electronic configurations of Q and R and determine their valences. Write the molecular formula of the compound formed when P and S combine.
Arrange the following in increasing order of property indicated
SiO2, P2O5, SO3, Cl2O7 (acidic nature)
Choose the most appropriate answer from the following list of oxides which fit the description.
An amphoteric oxide.
Arrange the following as per instruction given in the bracket.
Mg, Cl, Na, S, Si (decreasing order of atomic size)
An element A has 2 electrons in its fourth shell. State:
is it an oxidising or reducing agent
