Advertisements
Advertisements
Question
In solid state \[\ce{PCl5}\] is a ______.
Options
covalent solid
octahedral structure
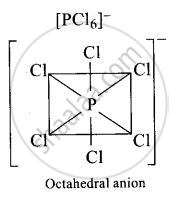
ionic solid with \[\ce{[PCl_{6}]^{+}}\] octahedral and \[\ce{[PCl_{4}]^{-} tetrahedra}\]
ionic solid with \[\ce{[PCl_{4}]^{+}}\] octahedral and \[\ce{[PCl_{6}]^{-} tetrahedra}\]
Solution
In solid state \[\ce{PCl5}\] is a ionic solid with \[\ce{[PCl_{4}]^{+}}\] octahedral and \[\ce{[PCl_{6}]^{-} tetrahedra}\].
Explanation:

APPEARS IN
RELATED QUESTIONS
Fluorine is a stronger oxidising agent than chlorine. Why?
Give two examples to show the anomalous behaviour of fluorine.
With what neutral molecule is ClO− isoelectronic? Is that molecule a Lewis base?
Cl2 acts as a bleaching agent.
Tincture of iodine is ____________.
Which one has the lowest boiling point?
Which of the following is isoelectronic pair?
Which of the following statements are true?
(i) Only type of interactions between particles of noble gases are due to weak dispersion forces.
(ii) Ionisation enthalpy of molecular oxygen is very close to that of xenon.
(iii) Hydrolysis of XeF6 is a redox reaction.
(iv) Xenon fluorides are not reactive.
Solubility of iodine in water may be increase by adding
Statement I: Acid strength increases in the order given as HF << HCl << HBr << HI.
Statement II: As the size of the elements F, Cl, Br, and I increase down the group, the bond strength of HF, HCl, HBr, and HI decreases and so the acid strength increases.
In the light of the above statements, choose the correct answer from the options given below.
