Advertisements
Advertisements
Question
Light of wavelength 3000Å falls on a metal surface having work function 2.3 eV. Calculate the maximum velocity of ejected electrons
(Planck's constant h = 6.63 x 10-34 J.s., Velocity of light c = 3 x 108 m/s, mass of an electron = 9.1 x 10-31 kg)
Solution
Given: λ = 3000 Å = 3 x 10–7 m, me = 9.1 10–31 kg,
Φo = 2.3 eV, h = 6.63 10–34 Js, c = 3 108 m/s
To find: Maximum velocity (vmax)
Formula: (K.E)max = `(hc)/λ - Φ_o`
Calculation: From formula,

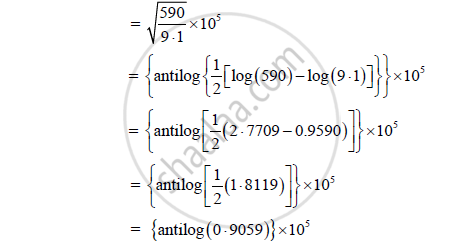
= 8.052 x 105 m/s
The maximum velocity of electron is 8.052 x 105 m/s
APPEARS IN
RELATED QUESTIONS
Light of a certain wavelength has a wave number `barv` in vacuum. Its wave number in a medium of refractive index n is _______.
Find the ratio of longest wavelength in Paschen series to shortest wavelength in Balmer series.
In hydrogen atom Balmer series is obtained when the electron jumps from ............ .
(a) higher orbit to first orbit
(b) first orbit to a higher orbit
(c) higher orbit to the second orbit
(d) second orbit to a higher orbit
What is de Broglie wavelength of an electron accelerated through 25000 volt?
