Advertisements
Advertisements
Question
Match the statement given in Column I with the phenomenon given in Column II.
| Column I | Column II |
| (i) Dispersion medium moves in an electric field | (a) Osmosis |
| (ii) Solvent molecules pass through semi permeable membrane towards solvent side |
(b) Electrophoresis |
| (iii) Movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes |
(c) Electroosmosis |
| (iv) Solvent molecules pass through semi permeable membranes towards solution side |
(d) Reverse osmosis |
Solution
| Column I | Column II |
| (i) Dispersion medium moves in an electric field | (c) Electroosmosis |
| (ii) Solvent molecules pass through semi permeable membrane towards solvent side |
(d) Reverse osmosis |
| (iii) Movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes |
(b) Electrophoresis |
| (iv) Solvent molecules pass through semi permeable membranes towards solution side |
(a) Osmosis |
Explanation:
(i) When electrophoresis i.e., movement of particles is prevented by some suitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed Electroosmosis.
(ii) Solvent molecules pass through semi-permeable membrane towards solvent side is termed as reverse osmosis.
(iii) When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode. The movement of colloidal particles under an applied electric potential is called electrophoresis.
(iv) Solvent molecules pass through semipermeable membrane towards solution side is termed as osmosis.
APPEARS IN
RELATED QUESTIONS
Out of MgCl2 and AlCl3, which one is more effective in causing coagulation of negatively charged sol and why?
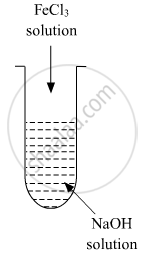
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
Which of the following will show Tyndall effect?
How does it become possible to cause artificial rain by spraying silver iodide on the clouds?
Why do we add alum to purify water?
What is Gold number?
Tyndall effect is observed in
Gold number of protective colloids A, Band Dare 0.50, 0.01 and 0.10 and 0.005. The correct order of their protecting power is
Define the following term:
Coagulation
Lyophilic sols are more stable than lyophobic sols because ______.
