Advertisements
Advertisements
Question
Which of the following will show Tyndall effect?
Options
Aqueous solution of soap below critical micelle concentration.
Aqueous solution of soap above critical micelle concentration.
Aqueous solution of sodium chloride.
Aqueous solution of sugar.
Solution
Aqueous solution of soap above critical micelle concentration.
Explanation:
Aqueous solution of soap above critical micelle concentration leads to the formation of colloidal solution. Tyndall effect is a characteristic of colloidal solution in which colloidal particles show a coloured appearance when sunlight is passes through it and seen perpendicularly.
APPEARS IN
RELATED QUESTIONS
Explain what is observed An electrolyte, NaCl is added to hydrated ferric oxide sol.
Explain what is observed Electric current is passed through a colloidal sol?
Explain the terms Coagulation
What happens when persistent dialysis of a colloidal solution is carried out?
Answer the following question.
Why are medicines more effective in the colloidal state?
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
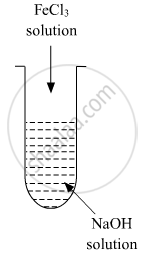
The right option for the statement “Tyndall effect is exhibited by”, is ______.
What is Electrophoresis?
Why a negatively charged sol is obtained when AgNO3 solution is added to KI solution?
Which of the following is a correct statement?
