Advertisements
Advertisements
Question
Name the substance obtained by the action of chlorine on solid (dry) slaked lime.
Solution
Bleaching powder, or calcium oxychloride (CaOCl2), is obtained by the action of chlorine on solid (dry) slaked lime.
APPEARS IN
RELATED QUESTIONS
Give the molecular formula of bleaching powder.
Which compound of calcium is used for disinfecting drinking water supply?
Name one compound of calcium which is used for removing the colour of a coloured cloth.
Which is the real bleaching agent present in bleaching powder?
State two important uses of bleaching powder.
Give a scientific explanation.
Bleaching powder has the odour of chlorine.
Brine is an ____________.
The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that gas with bleaching abilities was released.
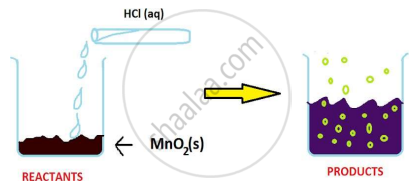
The chemical reaction between MnO2 and HCl is an example of:
Chlorine gas reacts with ____________ to form bleaching powder.
Assertion (A): Reaction of Quicklime with water is an exothermic reaction.
Reason (R): Quicklime reacts vigorously with water releasing a large amount of heat.
