Advertisements
Advertisements
Question
PH3 forms bubbles when passed slowly in water but NH3 dissolves. Explain why?
Solution
In PCl5, phosphorus undergoes sp3d hybridisation and a trigonal bipyramidal configuration comes into existence.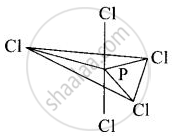
The equatorial P – Cl bonds are equivalent while two axial bonds are different and larger than equatorial bonds.
APPEARS IN
RELATED QUESTIONS
Account for the following : Solid PCl5 is ionic in nature.
Complete the following equations: Ag+PCl5 →
What happens when PCl5 is heated?
Write a balanced equation for the hydrolytic reaction of PCl5 in heavy water.
Can PCl5 act as an oxidising as well as a reducing agent? Justify.
Complete and balance the following equations:
PCL3 + H2O →
PCl5 is possible but NCl5 does not exist:
\[\ce{SF6}\] is known but \[\ce{SCl6}\] is not. Why?
White phosphorus reacts with chlorine and the product hydrolyses in the presence of water. Calculate the mass of \[\ce{HCl}\] obtained by the hydrolysis of the product formed by the reaction of 62 g of white phosphorus with chlorine in the presence of water.
What is the smoke screen?
