Advertisements
Advertisements
Question
In \[\ce{PCl5}\], phosphorus is in sp3d hybridised state but all its five bonds are not equivalent. Justify your answer with reason.
Solution
In \[\ce{PCl5}\], phosphorus undergoes sp3d hybridisation and a trigonal bipyramidal configuration comes into existence.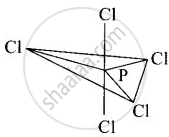
The equatorial \[\ce{P - Cl}\] bonds are equivalent while two axial bonds are different and larger than equatorial bonds.
APPEARS IN
RELATED QUESTIONS
Account for the following: Reducing character increases from NH3 to BiH3.
Write main differences between the properties of white phosphorus and red phosphorus.
The group 15 element having inner electronic configuration as of argon is-
(a) Phosphorous (z = 15)
(b) Antimony (z =51)
(c) Arsenic (z = 33)
(d) Nitrogen (z = 7)
Among the hydrides of Group-15 elements, which have the maximum basic character?
Among the hydrides of Group-15 elements, which have the maximum reducing character?
Give reasons When Cl2 reacts with the excess of F2, ClF3 is formed and not FCl3.
What is the action of sodium on arsenic.
The correct order of solubility in water for He, Ne, Ar, Kr, Xe is:
Collectively the elements of group 15 are called:
The enthalpy change of a reaction does not depends upon?
