Advertisements
Advertisements
Question
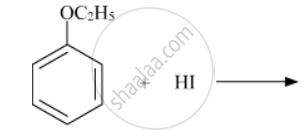
Predict the product of the following reaction:
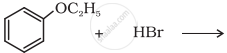
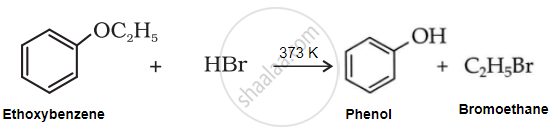
Solution
Due to resonance, the C6H5-O bond exhibits some double bond character, making it stronger than the O-C2H5 bond. Therefore, the weaker O-C2H5 bond undergoes cleavage, resulting in the formation of phenol and bromoethane.
APPEARS IN
RELATED QUESTIONS
Write the main product(s) in each of the following reactions:
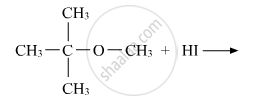
Predict the product of the following reaction:
\[\ce{CH3 - CH2 - CH2 - O - CH3 + HBr ->}\]
Predict the product of the following reaction:
\[\ce{(CH3)3C - OC2H5 ->[HI]}\]
Write the equation of the reaction of hydrogen iodide with 1-propoxypropane.
Write the equation of the reaction of hydrogen iodide with methoxybenzene.
Write the equation of the reaction of hydrogen iodide with benzyl ethyl ether.
Explain the fact that in aryl alkyl ethers
- the alkoxy group activates the benzene ring towards electrophilic substitution and
- it directs the incoming substituents to ortho and para positions in the benzene ring.
Write the mechanism of the reaction of HI with methoxymethane.
Write the product(s) in the following reaction
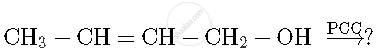
Write the formula of reagents used in the following reactions :
Bromination of phenol to 2,4,6-tribromophenol
Write the formula of reagents used in the following reactions :
Hydroboration of propene and then oxidation to propanol.
Write the structures of the main products in the following reactions :
Maximum number of H-bonds that can be formed by a water molecule is
Indicate the σ and π bond in the following molecule:
HCONHCH3
