Advertisements
Advertisements
Question
Rearrange the columns 2 and 3 so as to match with the column 1.
| Column 1 | Column 2 | Column 3 |
| i. Triad ii. Octave iii. Atomic number iv. Period v. Nucleus vi. Electron |
a. Lightest and negatively charged particle in all the atoms b. Concentrated mass and positive charge c. Average of the first and the third atomic mass d. Properties of the eighth element similar to the first e. Positive charge on the nucleus f. Sequential change in molecular formulae |
1.Mendeleev
2. Thomson
3. Newlands
4. Rutherford
5. Dobereiner
. Moseley
|
Solution
|
Column I
|
Column II
|
Column III
|
| i. Triad | c. Average of the first and the third atomic mass | 5. Dobereiner |
| ii. Octave | d. Properties of the eight-element similar to the first. | 3. Newlands |
| iii. Atomic Number | e. Positive charge on the nucleus. | 6. Moseley |
| iv. Period | f. Sequential change in molecular formulae | 1. Mendeleev |
| v. Nucleus | b. Concentrated mass and positive charge | 4. Rutherford |
| vi. Electron | a. Lightest and negatively charged particle in all the atoms | 2. Thomson |
APPEARS IN
RELATED QUESTIONS
Write the electronic configuration of two elements X and Y whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element X reacts with element Y. Draw the electron-dot structure of the product and also state the nature of the bond formed between both the elements.
An element X is in group 2 of the periodic table What will be the formula of its chloride?
An element X is in group 2 of the periodic table what will be the formula of its oxide?
An element X from group 2 of the periodic table reacts with an element Y from group 17 to form a compound.
(a) What is the nature of the compound formed?
(b) State whether the compound formed will conduct electricity or not.
(c) Give the formula of the compound formed.
(d) What is the valency of element X?
(e) How many electrons are there in the outermost shell of an atom of element Y?
The molecular formula of the chloride of element X is XCl. This compound is a solid having a high melting point. Which of the following elements be present in the same group as X.
(a) How is possible valency of an element determined from the electronic configuration of its atom ?
(b) Determine the valency of an element X whose atomic number is 15.
Elements belonging to the same group have the same valency.
Write the name.
The atom having smallest atomic radius from group 1.
Classify the following elements into Metals and Nonmetals.
S, Mg, Al, P, N, Na.
What is the atomic number of elements of period 3 and group 17 of the Periodic Table?
Which one of the following statements is not correct about the trends in the properties of the elements of a period on going from left to right?
Consider the following elements 20Ca, 8Or 18Ar, 16S, 4Be, 2He
Which of the above elements would you expect to be in group 16 of the Periodic Table?
Which of the following is a transition element?
Which of the following hydroxides is most basic?
Which of the following property will be common in group 1 elements?
An element with the atomic number 40 is placed in the period.
Which type of elements are poor conductors of heat and electricity?
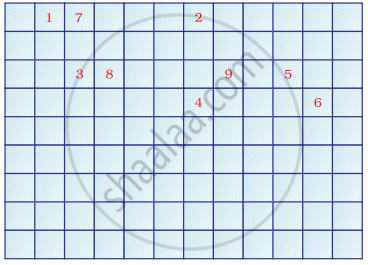
Complete the following cross word puzzle
Across:
(1) An element with atomic number 12.
(3) Metal used in making cans and member of Group 14.
(4) A lustrous non-metal which has 7 electrons in its outermost shell.
Down:
(2) Highly reactive and soft metal which imparts yellow colour when subjected to flame and is kept in kerosene.
(5) The first element of second Period
(6) An element which is used in making fluorescent bulbs and is second member of Group 18 in the Modern Periodic Table
(7) A radioactive element which is the last member of halogen family.
(8) Metal which is an important constituent of steel and forms rust when exposed to moist air.
(9) The first metalloid in Modern Periodic Table whose fibres are used in making bullet-proof vests
Complete the following triads by inserting the missing elements.
Li, ______, K
