Advertisements
Advertisements
Question
Rotation around carbon-carbon single bond of ethane is not completely free. Justify the statement.
Solution
Ethane contains carbon-carbon sigma (σ) bond. Electron distribution of the sigma molecular orbital is symmetrical around the intemuclear axis of the C – C bond which is not disturbed due to rotation about its axis. This permits free rotation around a C – C single bond. However, rotation around a C – C single bond is not completely free. It is hindered by a small energy barrier due to weak repulsive interaction between the adjacent bonds. Such a type of repulsive interaction is called torsional strain. Of all the conformations of ethane, the staggered form has the least torsional strain and the eclipsed form has the maximum torsional strain. The energy difference between the two extreme forms is of the order of 12.5 kJ mol–1, which is very small. It has not been possible to separate and isolate different conformational isomers of ethane.
APPEARS IN
RELATED QUESTIONS
How do you account for the formation of ethane during chlorination of methane?
Draw Newman and Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Rotation of one conformer by an angle between 0° to 60° generates ______.
The dihedral angle between the hydrogen atoms of 2 methyl groups in staggered conformation of ethane is
The dihedral angle of the least stable conformer of ethane is ______.
How many conformations does ethane have?
Consider the following carbocations:
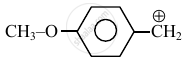
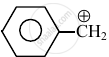
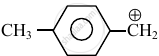
- \[\ce{CH3 - \overset{⊕}{CH2}}\]
The relative stabilities of these carbocations are such that:
