Advertisements
Advertisements
Question
Salt 'P', commonly used in bakery products, on heating gets converted into another salt 'Q' which itself is used for the removal of hardness of water and a gas 'R' is evolved. The gas 'R', when passed through freshly prepared lime water, turns milky. Identify 'P', 'Q' and 'R', giving a chemical equation for the justification of your answer.
Solution
Salt 'P', commonly used in bakery products is sodium hydrogen carbonate also known as baking soda. On heating, it gets converted into another salt 'Q' i.e. sodium carbonate which is used for the removal of hardness of water and the gas 'R' evolved is carbon dioxide.
\[\ce{\underset{\text{sodium hydrogen carbonate}}{2NaHCO3}->[heat]\underset{\text{sodium carbonate}}{Na2CO3}+\underset{\text{carbon}}{CO2}+\underset{\text{water}}{H2O}}\]
The gas 'R' i.e. CO2 , when passed through freshly prepared lime water, turns milky due to the formation of calcium carbonate.
\[\ce{\underset{\text{calcium hydroxide}}{Ca(OH)2}+CO2-> \underset{\text{calcium carbonate}}{CaCO3}+H2O}\]
APPEARS IN
RELATED QUESTIONS
Name the substance which on treatment with chlorine yields bleaching powder?
What will happen if a solution of sodium hydrocarbonate is heated? Give the equation of the reaction involved.
Give two important uses of baking soda.
Complete and balance the following chemical equations:
`NaHCO_3` 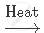
What happens when a cold and concentrated solution of sodium chloride reacts with ammonia and carbon dioxide? Write the chemical equation of the reaction which takes place.
Explain the action of baking powder in the making of cake (or bread). Write equation of the reaction involved.
The formula of baking soda is:
(a) K2CO3
(b) KHCO3
(c) NaHCO3
(d) Na2CO3
A person found that the cake prepared by him is hard and small in size. Which ingredient has he forgotten to add that would have caused the cake to rise and become light? Explain your answer.
Write the chemical formula.
Baking soda
Baking soda is a mixture of:
