Advertisements
Advertisements
Question
State how electrons are distributed in an atom. Explain in brief the rules which govern their distribution.
Solution
(a) Electrons revolve around the nucleus in imaginary paths called shells or orbits. Shells start from the nucleus to outwards.
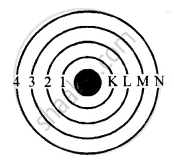
Rules: The maximum number of electrons in a shell is given by 2n2. Where n is the number of shell i.e. 1st shell can have a maximum of 2 electrons.
2n2 = 2(1)2 = 2 × 1 = 2
2nd shell can have maximum of 8 electrons
2n2 = 2(2)2 = 2 × 4 = 8
3rd shell can have maximum of 18 electrons
2n2 = 2 (3)2 = 2 × 9 = 18 and so on….
(b) Outer most orbit cannot have more than 8 electrons and 18 in penultimate orbit.
(c) A new shell cannot start until previous is filled completely.
APPEARS IN
RELATED QUESTIONS
Write true or false for the following statement
The central pad of the atom is called nucleus.
Give the following a suitable word/phrase
The sub-atomic particle with negative charge and negligible mass.
What are the two main parts of which an atom is made of?
WRITE SHORT ANSWER
Name the isotopes of hydrogen.
WRITE SHORT ANSWER
What is the number of neutrons present in a potassium atom ?
State whether the following statement is true or false :
The anode rays obtained from all the gases consist of positively charged particles called protons.
Compare an electron and a proton in respect of mass and charge.
The particle not present in an ordinary hydrogen atom is :
Name the three fundamental particles of an atom.
Explain how the modern atomic theory contradicted Dalton’s atomic theory.
