Advertisements
Advertisements
Question
State your observation When moist starch iodide paper is introduced into chlorine gas.
Solution
When moist starch iodide paper is introduced into chlorine gas, chlorine oxidizes iodide to iodine, which shows up as blue when complexed with starch.
APPEARS IN
RELATED QUESTIONS
The acid on mixing with silver nitrate solution produces a white precipitate which is soluble in excess ammonium hydroxide
For the preparation of hydrochloric acid in the laboratory:
What arrangement is done to dissolve hydrogen chloride gas in water?
Write an equation for the reaction of hydrochloric acid on caustic soda solution.
Write an equation for the reaction of aqueous hydrochloric acid on lead nitrate solution.
How will the action of dilute hydrochloric acid enable you to distinguish between sodium thiosulphate and sodium sulphite?
Convert two soluble metallic nitrates to insoluble metallic chlorides using \[\ce{dil. HCl}\].
What is the function of HCI in preparation of aqua-regia?
Identify the salts P and Q from the observation given below:
When dilute HCl is added to a salt Q, a brisk effervescence is produced and the gas turns lime water milky. When NH4OH soltion is added to the above mixture (after adding dilute HCl), it produces a white precipitate which is soluble in excess NH4OH solution.
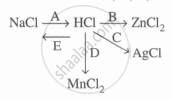
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D, and E.
State which of the two - a solution of HCl in water or in toluene is an electrolyte. Explain.
