Advertisements
Advertisements
Question
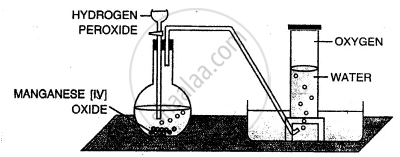
Taking hydrogen peroxide, how would you prepare oxygen in the laboratory ?
Solution
Take manganese dioxide in a round bottom flask and add hydrogen peroxide drop by drop to it, which acts ; a catalyst as shown in the figure. Collect oxygen by downward displacement of water.
APPEARS IN
RELATED QUESTIONS
What happens when mercuric oxide is heated?
Fill in the blank :
____________ discovered the oxygen gas.
Statement given below is incorrect. Write the correct statement :
The percentage of oxygen in air by volume, varies from 21% to 22%.
Fill in the blank:
Oxygen is ___________ to litmus testing.
Fill in the blank space by choosing the correct word from the given list.
List: sulphurous, nitric acid, red lead oxide paint, oxygen, phosphoric
Potassium nitrate crystals on heating strongly yield potassium nitrite and ________ gas.
Tick (√ ) the most appropriate answer.
The catalyst used in the preparation of oxygen from hydrogen peroxide is :
Write fully balanced equation for the following :
SO2 + H2O →
Give reasons for the following:
patients suffering from lung problems are kept in an oxygen tent.
Give reason for the following:
Oxidation of sulphur results in a product which turns moist blue litmus red.
Mention the physical properties of oxygen.
