Advertisements
Advertisements
Question
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
| The carbon-oxygen double bond is polarised in aldehydes and ketones due to higher electronegativity of oxygen relative to carbon. Therefore, they undergo nucleophilic addition reactions with a number of nucleophiles such as HCN, NaHSO3, alcohols, ammonia derivatives and Grignard reagents. Aldehydes are easily oxidised by mild oxidising agents as compared to ketones. The carbonyl group of carboxylic acid does not give reactions of aldehydes and ketones. Carboxylic acids are considerably more acidic than alcohols and most of simple phenols. |
Answer the following:
(a) Write the name of the product when an aldehyde reacts with excess alcohol in the presence of dry HCl. (1)
(b) Why carboxylic acid is a stronger acid than phenol? (1)
(c) (i) Arrange the following compounds in increasing order of their reactivity towards CH3MgBr: (1)
CH3CHO, \[\begin{array}{cc}
\ce{(CH3)3C-C-CH3}\\
\phantom{....}||\\
\phantom{....}\ce{O}
\end{array}\], \[\begin{array}{cc}
\ce{CH3-C-CH3}\\
||\\
\ce{O}
\end{array}\]
(ii) Write a chemical test to distinguish between propanal and propanone. (1)
OR
(c) Write the main product in the following: (2)
| (i) | 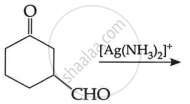 |
| (ii) | 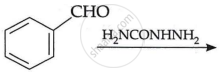 |
Solution
(a) When an aldehyde interacts with an excess of alcohol in the presence of dry HCl, alkoxyalcohol intermediates known as hemiacetals are created, which then react with one more molecule of alcohol to give acetal, a gem-dialkoxy chemical.
(b) The carboxylate ion, the conjugate base of carboxylic acid, is stabilised by two equivalent resonance structures in which the negative charge resides at the more electronegative oxygen atom. The phenoxide ion conjugate base of phenol possesses non-equivalent resonance structures with a negative charge at the less electronegative carbon atom. As a result, resonance in phenoxide ions is less essential than in carboxylate ions. As a result, carboxylic acids are more acidic than phenols.
(c) (i)
\[\begin{array}{cc}
\ce{(CH3)3C-C-CH3 < CH3-C-CH3 < CH3CHO}\\
\phantom{.}||\phantom{...............}||\phantom{.........}\\
\phantom{}\ce{O}\phantom{..............}\ce{O}\phantom{.........}
\end{array}\]
(ii) Even moderate oxidising agents like Tollen's reagent and Fehling's reagent oxidise aldehydes, generating carboxylic acid. Propanal, on the other hand, does not pass Tollen's test.
OR
(c)
| (i) | 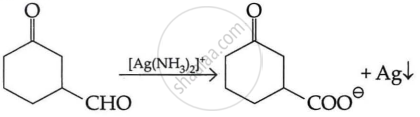 |
| (ii) |  |
APPEARS IN
RELATED QUESTIONS
Write the products formed when CH3CHO reacts with the following reagents : H2N – OH
Arrange the following compound in increasing order of its reactivity in nucleophilic addition reactions.
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint: Consider steric effect and electronic effect.
What is meant by the following term? Give an example of the reaction in the following case.
Acetal
Write balanced chemical equations for action of ammonia on - acetone
Write balanced chemical equations for action of ammonia on - acetone
How will you convert sodium acetate to methane?
In the following reaction
\[\ce{Carbonyl compound + MeOH <=>[HCl] acetal}\]
Rate of the reaction is the highest for ______.
The product of following reaction is
\[\ce{CH3 - CH = CH - CH2 - CHO ->[i) LiAlH4][ii) H3O+]}\] ______?
Draw structures of the following derivatives.
Acetaldehydedimethylacetal
Give an example of the reaction in the following case.
Oxime
