Advertisements
Advertisements
Question
How will you convert sodium acetate to methane?
Solution
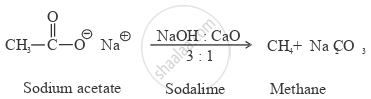
APPEARS IN
RELATED QUESTIONS
Write balanced chemical equations for action of ammonia on - formaldehyde
How are the following compounds prepared?
benzaldehyde from benzene
Write the products formed when CH3CHO reacts with the following reagents : HCN
Predict the product of the following reaction:
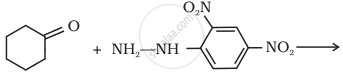
Predict the product of the following reaction:
\[\begin{array}{cc}
\phantom{..............}\ce{O}\\
\phantom{..............}||\\
\ce{R - CH = CH - CHO + NH2 - C - NH - NH2 ->[H+]}\end{array}\]
What is meant by the following term? Give an example of the reaction in the following case.
Hemiacetal
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
PhMgBr and then H3O+
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
Excess ethanol and acid
Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
Give plausible explanation for the following:
Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6 trimethylcyclohexanone does not.
Give plausible explanation for the following:
There are two −NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
Write the main product formed when propanal reacts with the following reagents:
H2N- NH2 followed by heating with KOH in ethylene glycol.
Which of the following compounds is most reactive towards nucleophilic addition reactions?
Which of the following is the correct representation for intermediate of nucleophilic addition reaction to the given carbonyl compound (A):

(i) 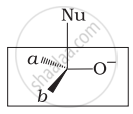
(ii) 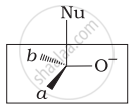
(iii) 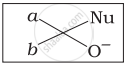
(iv) 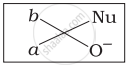
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Which of the following has the most acidic hydrogen?
A Idol condensation will not be observed in
The most stable reagent for the conversion of R – CH2OH → RCHO is
Which among the following is most reactive to give nucleophilic addition?
What is the action of sodium hypoiodite on acetone?
What happens when propanone is treated with CH3MgBr and then hydrolysed?
Which will undergo faster nucleophilic addition reaction?
Acetaldehyde or Propanone
In the following reaction
\[\ce{Carbonyl compound + MeOH <=>[HCl] acetal}\]
Rate of the reaction is the highest for ______.
The product of following reaction is
\[\ce{CH3 - CH = CH - CH2 - CHO ->[i) LiAlH4][ii) H3O+]}\] ______?
Draw structures of the given derivatives.
The ethylene ketal of hexan-3-one
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structures of the given derivatives.
The ethylene ketal of hexan-3-one
