Advertisements
Advertisements
Question
Give plausible explanation for the following:
There are two −NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
Solution
Semicarbazide only has one of the two −NH2 groups resonance and this group is directly attached to the carbonyl-carbon atom.
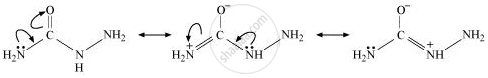
Therefore, the electron density on −NH2 group involved in the resonance also decreases. As a result, it cannot act as a nucleophile. Since the other −NH2 group is not involved in resonance, it can act as a nucleophile and attack carbonyl-carbon atoms of aldehydes and ketones to produce semicarbazones.
APPEARS IN
RELATED QUESTIONS
How are the following compounds prepared?
benzaldehyde from benzene
Predict the product of the following reaction:
\[\begin{array}{cc}
\phantom{..............}\ce{O}\\
\phantom{..............}||\\
\ce{R - CH = CH - CHO + NH2 - C - NH - NH2 ->[H+]}\end{array}\]
How will you bring about the following conversion in not more than two steps?
Bromobenzene to 1-Phenylethanol
How are the following compounds prepared?
acetophenone from benzene
Write balanced chemical equations for action of ammonia on - acetone
Which of the following has the most acidic hydrogen?
Acetaldehyde and acetone differ in their reaction with
What is the action of sodium hypoiodite on acetone?
What happens when propanone is treated with CH3MgBr and then hydrolysed?
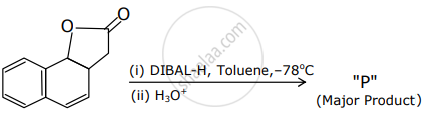
The product "P" in the above reaction is:
Aldehydes and ketones react with hydroxylamine to form ______.
Write the structure of the product formed when acetone reacts with 2, 4 DNP reagent.
Draw structure of the following derivative.
Acetaldehydedimethylacetal
Draw structures of the following derivatives.
Acetaldehydedimethylacetal
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Give an example of the reaction in the following case.
Oxime
Give an example of the reaction in the following case.
Imine
