Advertisements
Advertisements
Question
What happens when propanone is treated with CH3MgBr and then hydrolysed?
Solution
When propanone reacts with ethyl magnesium bromide (Grignard's reagent) and hydrolysed, it forms aldehyde.
\[\begin{array}{cc}
\ce{(CH3)2CO + H3CCH2MgBr ->[][H2O] CH2CH3}\\
\phantom{..........................}|\\
\phantom{....................................}\ce{(CH3)2COH + Mg(OH)Br}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
How are the following compounds prepared?
benzaldehyde from benzene
Predict the product of the following reaction:
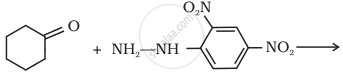
Give plausible explanation for the following:
Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6 trimethylcyclohexanone does not.
How are the following compounds prepared?
benzaldehyde from benzoyl chloride
Write the main product formed when propanal reacts with the following reagents:
H2N- NH2 followed by heating with KOH in ethylene glycol.
Which of the following compounds is most reactive towards nucleophilic addition reactions?
Alkenes 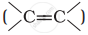 and carbonyl compounds
and carbonyl compounds 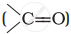 , both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
, both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
The increasing order of the following compounds towards HCN addition is:
| (i) | 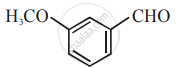 |
| (ii) | 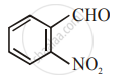 |
| (iii) | 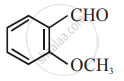 |
| (iv) | 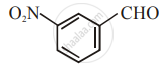 |
Out of p-tolualdehyde and p-nitrobenzaldehyde, which one is more reactive towards nucleophilic addition reactions, why?
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
