Advertisements
Advertisements
Question
Alkenes 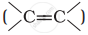 and carbonyl compounds
and carbonyl compounds 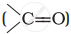 , both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
, both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
Solution
Carbonyl group is polar in nature. Due to larger electronegativity of oxygen as compared to carbon, carbon acquires partial positive charge while O acquires partial negative charge.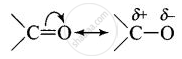
Because of slight positive charge on C atom, it is attacked by nucleophiles and, therefore, undergoes nucleophilic addition reaction.
Ethylenic double bond is a non-polar bond and is a source of electrons. Therefore, it is attacked by electrophiles and undergoes electrophilic addition reactions.
APPEARS IN
RELATED QUESTIONS
Arrange the following compound in increasing order of its reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone.
Hint: Consider steric effect and electronic effect.
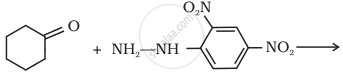
Predict the product of the following reaction:
What is meant by the following term? Give an example of the reaction in the following case.
Acetal
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
PhMgBr and then H3O+
How are the following compounds prepared?
benzaldehyde from benzoyl chloride
Which one of the following gives only one monochloro derivative?
In the following reaction
\[\ce{Carbonyl compound + MeOH <=>[HCl] acetal}\]
Rate of the reaction is the highest for ______.
Aldehydes and ketones react with hydroxylamine to form ______.
Draw structure of the following derivative.
Acetaldehydedimethylacetal
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
