Advertisements
Advertisements
Question
Which of the following is the correct representation for intermediate of nucleophilic addition reaction to the given carbonyl compound (A):

(i) 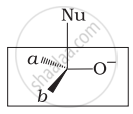
(ii) 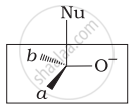
(iii) 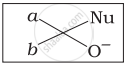
(iv) 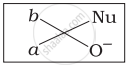
Solution
(i) 
(ii) 
Explanation:
The given compound is a planar molecule with sp2 hybridized carbon. Carbon atoms are attacked by nucleophiles. If the nucleophile approaches from the front, the (i) and (ii) molecules shift to the front and back positions, respectively and the carbon becomes tetrahedral. Another alternative is that (i) and (ii) molecules will be located above and below the plane, respectively. As a result, the choices (iii) and (iv) aren't represented as planar molecules.
APPEARS IN
RELATED QUESTIONS
Predict the product of the following reaction:
\[\begin{array}{cc}
\phantom{..............}\ce{O}\\
\phantom{..............}||\\
\ce{R - CH = CH - CHO + NH2 - C - NH - NH2 ->[H+]}\end{array}\]
What is meant by the following term? Give an example of the reaction in the following case.
Oxime
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
PhMgBr and then H3O+
Complete the synthesis by giving missing starting material, reagent or product.
\[\ce{C6H5CHO ->[H2NCONHNH2]}\]
Explain the mechanism of alkaline hydrolysis of tert-butyl bromide with energy profile diagram.
Write balanced chemical equations for action of ammonia on - acetone
Write the main product formed when propanal reacts with the following reagents:
2 moles of 3 CH OH in presence of dry HCl
Write a test to differentiate between pentan-2-one and pentan-3-one.
What is the action of sodium hypoiodite on acetone?
Which of the following is most reactive in nucleophilic addition reactions?
