Advertisements
Advertisements
Question
The element hydrogen is present in ______ organic compound.
Options
single
all double
ionic
carbon
give and take
hydrogen
multiple
share
most
covalent
Solution
The element hydrogen is present in most organic compound.
APPEARS IN
RELATED QUESTIONS
At least one carbon bond in an unsaturated hydrocarbon is ______.
What do you mean by catenation?
______ compounds decolourize bromine water.
Answer the following question using the data given below:
- Assertion: Alkanes are saturated hydrocarbons.
- Reason: Hydrocarbons consist of covalent bonds.
Complete and balance the following chemical equation:
C2H6 + O2 →
Dead and decaying plants and animals release ______ gas.
Which gas is used as fuels and solvents in the laboratory?
Hydrocarbons are less dense than ______.
Sewage sludge can also be decomposed by microorganisms to produce methane gas along with impurities like carbon dioxide and ______.
______ is used as fuel gas and propellant in aerosol sprays such as deodorants.
Match the following:
| 1. | Hydrocarbons | a. | Marsh gas |
| 2. | Methane | b. | Cleanest fuel |
| 3. | Butane | c. | Catenation |
| 4. | CNG | d. | Polystyrene |
Write any 2 properties of hydrocarbons.
The hydrocarbons containing carbon-carbon multiple bonds are called ______.
Identify saturated and unsaturated hydrocarbons from the given structural formula:
| \[\begin{array}{cc} \phantom{}\ce{H}\phantom{..}\ce{H}\phantom{}\\ \phantom{}|\phantom{...}|\phantom{}\\ \ce{H-C-C-H}\\ \phantom{}|\phantom{...}|\phantom{}\\ \phantom{}\ce{H}\phantom{..}\ce{H}\phantom{} \end{array}\] |
\[\begin{array}{cc} \phantom{..}\ce{H}\phantom{........}\ce{H}\phantom{.}\\ \phantom{.}\backslash\phantom{......}/\phantom{}\\ \ce{C = C}\\ \phantom{}/\phantom{......}\backslash\phantom{}\\ \phantom{}\ce{H}\phantom{........}\ce{H}\phantom{} \end{array}\] |
| (1) | (2) |
Draw electron dot structure for the following:
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{..}\ce{H}\phantom{}\\
\phantom{}|\phantom{...}|\phantom{}\\
\ce{H-C-C-H}\\
\phantom{}|\phantom{...}|\phantom{}\\
\phantom{}\ce{H}\phantom{..}\ce{H}\phantom{}
\end{array}\]
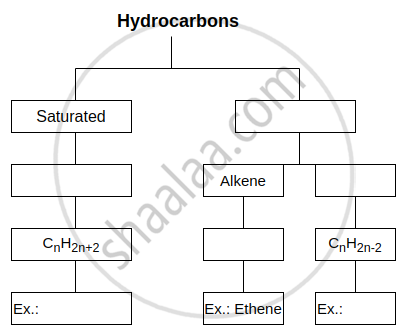
Complete the following flow chart:
Identify the hydrocarbon from the given electron-dot structure:
\[\begin{array}{cc}
\phantom{}\ce{H}\phantom{....}\ce{H}\phantom{}\\
\phantom{}\ce{H}\phantom{..}\ce{\text{}^\bullet_\bullet}\phantom{..}\ce{\overset{\bullet\phantom{.}\bullet}{\underset{\bullet\phantom{.}\bullet}{C}}}\phantom{..}\ce{\text{}^\bullet_\bullet}\phantom{..}\ce{\overset{\bullet\phantom{.}\bullet}{\underset{\bullet\phantom{.}\bullet}{C}}}\phantom{..}\ce{\text{}^\bullet_\bullet}\phantom{..}\ce{H}\phantom{}\\
\phantom{}\ce{H}\phantom{....}\ce{H}\phantom{}\\
\end{array}\]
