Advertisements
Advertisements
Question
The letters ‘D’ or ‘L’ before the name of a stereoisomer of a compound indicate the correlation of configuration of that particular stereoisomer. This refers to their relation with one of the isomers of glyceraldehyde. Predict whether the following compound has ‘D’ or ‘L’ configuration.
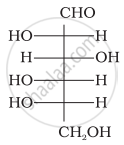
Solution
The letter 'D' or 'L" before the name of any compound indicate the relative configuration of a particular stereoisomer. This refers to their relation with a particular isomer of glyceraldehydes.
D' before the name of glucose represents the configuration whereas '(+)' represents dextrorotatory nature of the molecule. It may be remembered that 'D' and 'L' have no relation with the optical activity of the compound. For assigning the configuration of monosaccharides, it is the lowest asymmetric carbon atom which is compared. The given compound has L-configuration.
APPEARS IN
RELATED QUESTIONS
What are monosaccharides?
Draw ring structure of α - D - (+) - glucopyranose.
Write the name of two monosaccharides obtained on hydrolysis of lactose sugar.
Write the product obtained when D-glucose reacts with H2N − OH.
How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Which one of the following is a monosaccharide:
starch, maltose, fructose, cellulose
Assertion: All naturally occurring α-aminoacids except glycine are optically active.
Reason: Most naturally occurring amino acids have L-configuration.
Naturally occurring glucose is called as ______.
