Advertisements
Advertisements
Question
The nuclei of isotopes of a given element contain the same number of ______.
Options
Neutrons
Neutrinos
Positrons
Protons
MCQ
Fill in the Blanks
Solution
The nuclei of isotopes of a given element contain the same number of protons.
Explanation:
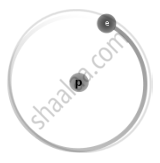
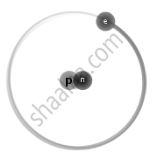
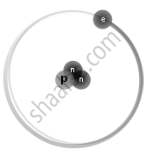
Isotopes are different neutron number variants of the same chemical element. Each atom in all isotopes of a specific element has the same number of protons. Example:
 |
 |
 |
| `"_1^1H` Protium |
`"_1^2H` Deuterium |
`"_1^3H` Tritium |
shaalaa.com
Is there an error in this question or solution?
