Advertisements
Advertisements
Question
The number of terminal oxygen atoms present in the product B obtained from the following reactions is:
\[\ce{FeCr2O4 + Na2CO3 + O2 -> A + Fe2O3 + CO2}\]
\[\ce{A + H^+ -> B + H2O + Na^+}\]
Options
2
4
6
8
MCQ
Solution
6
Explanation:
\[\ce{FeCr2O4 + Na2CO3 + O2 -> \underset{(A)}{Na2CrO4} + Fe2O3 + CO2}\]
\[\ce{\underset{(A)}{Na2CrO4} + H^+ -> \underset{(B)}{Na2Cr2O7} + Na^+ + H2O}\]
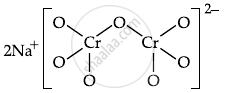
The number of terminal oxygen atoms present in the product B is 6.
shaalaa.com
Is there an error in this question or solution?
