Advertisements
Advertisements
Question
The radius of atom is of the order of 1 Å and the radius of nucleus is of the order of fermi. How many magnitudes higher is the volume of atom as compared to the volume of nucleus?
Solution
Radius of atom = 1 Å = 10–10 m
Radius of nucleus = 1 fermi = 10–15 m
Volume of atom = VA = `4/3 πR_A^3`
Volume of atom = `4/3 πR_A^3`
Volume of nucleus VN = `4/3 πR_N^3`
`V_A/V_N = (4/3 πR_A^3)/(4/3 πR_N^3)`
= `(R_A/R_N)^3`
= `(10^-10/10^-15)^3`
= 1015
APPEARS IN
RELATED QUESTIONS
A hand span is a reliable measure of length.
While measuring the length of a sharpened pencil, the reading of the scale at one end is 2.0 cm and at the other end is 12.1 cm. What is the length of the pencil?
Fill in the following chart.
| Property | Definition | Basic Unit | Instrument used for measuring |
| Length | |||
| Mass | |||
| Volume | |||
| Time |
Assertion (A): Distance between two celestial bodies is measured in terms of light year.
Reason (R): The distance travelled by the light in one year is one light year.
What is the length of a 100 rupee note? Guess. Now measure it using a scale.

Arbaz plans to tile his kitchen floor with green square tiles. Each side of the tile is 10 cm. His kitchen is 220 cm in length and 180 cm wide. How many tiles will he need?

Can you think of how to cut a postcard so that you can pass through it?

There are two beautiful lakes near a village. People come for boating and picnics in both the lakes. The village Panchayat is worried that with the noise of the boats the birds will stop coming. The Panchayat wants motorboats in only one lake. The other lake will be saved for the birds to make their nests.
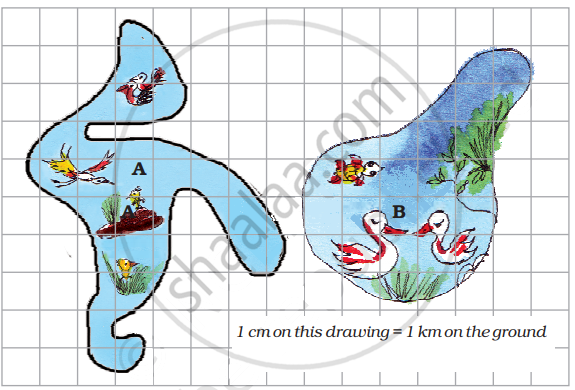
- What is the length of the boundary of lake B in the drawing?
What is the unit of measurements of very small lengths?
SI unit of electric current is ______.
