Advertisements
Advertisements
Question
Just as precise measurements are necessary in science, it is equally important to be able to make rough estimates of quantities using rudimentary ideas and common observations. Think of ways by which you can estimate the following (where an estimate is difficult to obtain, try to get an upper bound on the quantity):-
the number of air molecules in your classroom.
Solution 1
Let the volume of the room be V.
One mole of air at NTP occupies 22.4 l i.e., 22.4 × 10–3 m3 volume.
Number of molecules in one mole = 6.023 × 1023
∴Number of molecules in room of volume V
=`(6.023xx10^(23))/(22.4xx10^(-3))xxV`
= 134.915 × 1026 V
= 1.35 × 1028 V
Solution 2
We can determine the volume of the class-room by measuring its length, breadth and height. Consider a class room of size 10 m x 8 m x 4 m.
Volume of this room is 320 m3.
We know that 22.4l or 22.4 x 10-3 m3 of air has 6.02 x 1023 molecules (equal to Avogadro’s number).
Number of molecules of air in the class room =(6.02 x 1023 /22.4 x 10-3 ) x 320 =8.6 x 1027
APPEARS IN
RELATED QUESTIONS
Write true or false.
Every measurement involves two things – a number and a unit.
Ten millimetre makes one centimetre.
Complete the analogy.
Height of a person: cm :: Length of your sharpened pencil lead:______?
Can you find the diameter of a thin wire of length 2 m using the ruler from your instrument box?
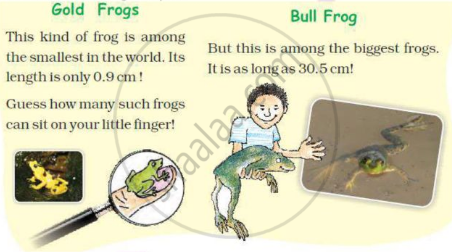
Have you seen frogs? Where? How many different types of frogs have you seen? Are all the frogs of the same length? Here are two interesting examples.
The fencing of a square garden is 20 m in length. How long is one side of the garden?
There are two beautiful lakes near a village. People come for boating and picnics in both the lakes. The village Panchayat is worried that with the noise of the boats the birds will stop coming. The Panchayat wants motorboats in only one lake. The other lake will be saved for the birds to make their nests.
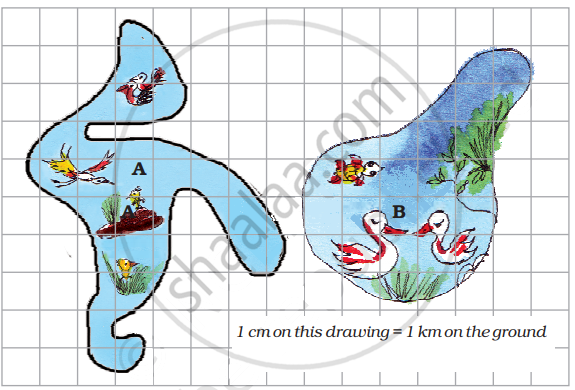
- What is the length of the boundary of lake B in the drawing?
In which SI unit, you can measure your height?
What is the unit of measurements of very small lengths?
How many astronomical units (A.U.) make 1 parsec?
