Advertisements
Advertisements
Question
Under what conditions does ethane get converted to ethyl alcohol?
Solution 1
Ethane is mixed with oxygen and is passed through a hot copper tube; it gets oxidized to ethyl alcohol.
\[\ce{2C2H6 + O2 ->[Cu][200^\circ C] 2C2H5OH}\]
Solution 2
Step 1:
C2H6 is converted into C2H5Cl
C2H6 + Cl2 → C2H5Cl + HCl
Step 2:
By hydrolysis of Ethyl Halides
\[\ce{\underset{[Ethyl chloride]}{C2H5Cl} + NaOH -> \underset{[Ethyl alcohol]}{C2H5OH} + NaCl}\]
APPEARS IN
RELATED QUESTIONS
A student takes about 6 ml of distilled water in each of the four test tubes P, Q, R and S. He then dissolves an equal amount of four different salts namely, sodium chloride in 'P', potassium chloride in 'Q', calcium chloride in 'R' and magnesium chloride in 'S'. Next, he then adds 10 drops of soap solution to each test tube and shakes its contents. The test tubes in which scum (insoluble substance) is formed with soap are:
(A) P and Q
(B) Q and R
(C) R and S
(D) Q and S
Write the IUPAC names, common names and formulae of the first two members of the homologous series of carboxylic acids.
How does ethanoic acid react with sodium hydrogen carbonate? Give equation of the reaction which takes place.
A neutral organic compound is warmed with some ethanoic acid and a little of conc. H2SO4. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound?
An organic compound A (molecular formula C2H4O2) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of a little of concentrated sulphuric acid forms a sweet-smelling compound D (molecular formula C3H6O2). Compound D on treatment with NaOH solution gives back B and C. Identify A, B, C and
Acetic acid smells like:
(1) a banana
(2) vinegar
(3) an orange
(4) a lemon
State are relevant observations for following reactant:
Addition of ethyl alcohol to acetic acid in presence of conc. H2SO4
The reaction between acetic acid and sodium carbonate is shown in the following figure.
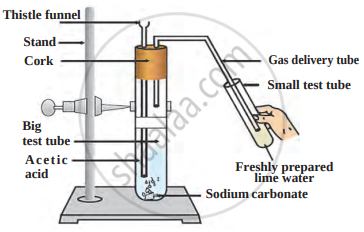
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
Observe the diagram given below and answer the questions:
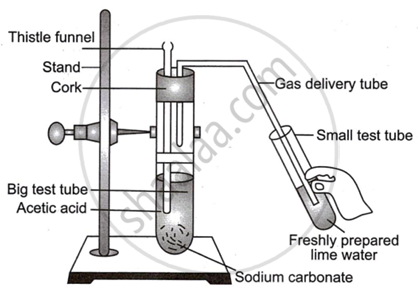
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
