Advertisements
Advertisements
Question
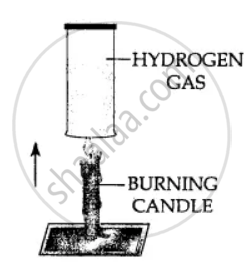
Using a burning candle and a jar of hydrogen – how would you prove experimentally that Hydrogen is a combustible gas.
Solution
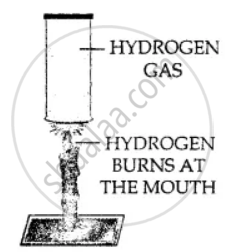
Hydrogen gas is combustible and bums at the mouth of the gas jar. When a burning candle is brought near the mouth of hydrogen in gas jar.
APPEARS IN
RELATED QUESTIONS
Give a balanced chemical equation for the reaction.
State four uses of hydrogen:
MULTIPLE CHOICE QUESTIONS
Which is the lightest of all elements?
- hydrogen
- helium
- lithium
- none of these
Give the balanced equation of the reaction.
Give balanced equation for the following conversion:
Nitrogen to a basic gas – using hydrogen.
Give reason for the following:
The reaction of chlorine with hydrogen sulphide is deemed a redox reaction.
Give a chemical test to distinguish between the following gases:
Ammonia and hydrogen chloride
Describe one chemical test applied to the following gases, which would enable you to distinguish between them :‘carbon monoxide and hydrogen’.
Complete and balance the following equations:
Al + NaOH + ____ → ____ + ____
Select the correct answer from the symbol in the bracket.
The element, which like hydrogen has one valence electron.
