Advertisements
Advertisements
Question
Using IUPAC norms write the systematic name of the following:
[Pt(NH3)2Cl(NH2CH3)]Cl
Solution
Diamminechlorido(methylamine)platinum(II) chloride
APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of [ Co(NO2)3(NH3)3 ].
Write the IUPAC name of 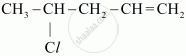
IUPAC name of K4[Fe(CN)6] is
Write the IUPAC name of the given compound:
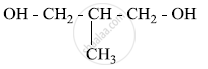
Write down the IUPAC name of the following complex: [Cr(NH3)2Cl2(en)]Cl (en = ethylenediamine)
Write down the IUPAC name of the complex [Pt(en)2Cl2]2+. What type of isomerism is shown by this complex?
Write the IUPAC name of the complex [Cr(NH3)4 Cl2]Cl.
Write the IUPAC name of the following coordination compound:
K2[PdCl4]
Specify the oxidation number of the metal in the following coordination entity:
[CoBr2(en)2]+
Write the IUPAC name of the following coordination compound:
K3[Fe(CN)6]
Write the IUPAC names of the following coordination compounds:
[CoBr2(en)2]+, (en = ethylenediamine)
How will you convert the following?
Nitrobenzene into aniline
How will you convert the following?
Aniline into N−phenylethanamide
Name the type of isomerism shown by the following compounds:
[CU(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
Name the type of isomerism shown by the following compounds:
[Co(Pn)2Cl2]+ and [Co(en)2Cl2]+
Which of the following is paramagnetic?
The formula Co(NH3)5CO3Cl could represent a carbonate or a chloride. Write the structures and names of possible isomers.
The complex Hg[Co(CNS)4] is correctly named as ______.
