Advertisements
Advertisements
Question
What happens when a rod of zinc metal is dipped into a solution of copper sulphate? Give chemical equation of the reaction involved.
Solution
When a zinc rod is dipped in a solution of copper sulphate, zinc, being a more reactive metal than copper, will displace copper from copper sulphate solution. The blue colour of the copper sulphate solution gradually fades due to the formation of the green-coloured zinc sulphate. The red-brown colour of copper will be deposited on the zinc rod.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
APPEARS IN
RELATED QUESTIONS
A copper coin is kept immersed in a solution of silver nitrate for some time. What will happen to the coin and the colour of the solution?
Complete and balance the following equation:
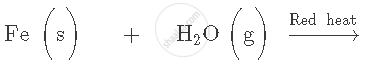
Why is white phosphorus kept immersed under water?
Describe the reaction of potassium with water. Write the equation of the reaction involved.
What is the action of water on aluminium ? Write equation of the chemical reaction involved.
Write the equation for the reaction of Magnesium with dilute hydrochloric acid.
Name the products formed. Also indicate the physical states of all the substances involved.
The elements whose oxides can turn litmus solution blue are:
(a) carbon and sulphur
(b) sodium and carbon
(c) potassium and magnesium
(d) magnesium and sulphur
Answer the following question:
An element X on reacting with oxygen forms an oxide X2O. This oxide dissolves in water and turns red litmus blue. State whether element X is metal or a non - metal. Explain with a proper example.
Metals are generally hard. Which of the following metals is an exception and can be cut with a knife?
Which of the following property is not responsible for copper to be used as electrical conduction wires?
