Advertisements
Advertisements
Question
What is the difference between molality and molarity?
Solution
The number of moles of a substance (known as the solute) dissolved in precisely 1 litre of a solution is known as molarity (solvent and solute combined). As a result, the formula for estimating molarity is as follows:
Molarity = `"Number of moles of solute"/"Volume in litre"`
The term molarity is also used to refer to molar concentration. As a result, molar concentration measurement is based on the volume of liquid in which a substance is dissolved. It's vital to remember that the volume is in litres, so if we have volume in mL we need to convert that in litres,
Molality is the number of moles of substance (also known as the solute) found in a given mass of solvent (in Kg) in which it is dissolved
Molality is calculated by using the formula.
Major differences between Molarity and Molality ar given as follows:
1) Molarity is the concentration of a material determined as the number of moles of solute dissolved in poe litre of solution, whereas morality is the concentration calculated as the number of moles of solute found in one kilogram of solvent.
2) Molality is denoted by the symbol m, whereas molarity is denoted by the symbol M.
3) The molarity formula is moles per litre, but the morality is formula is moles per kilogram.
4) Molarity is impacted by temperature changes, whereas morality is affected by temperature changes.
5) changes in pressure affect molarity, but they do not affect molality.
6) Molarity can lead to an imprecise and inaccurate concentration, whereas molarity can lead to an accurate and precise concentration measurement.
APPEARS IN
RELATED QUESTIONS
Calculate the amount of carbon dioxide that could be produced when 1 mole of carbon is burnt in air.
If the density of methanol is 0.793 kg L–1, what is its volume needed for making 2.5 L of its 0.25 M solution?
Explain the term mole fraction
Solve the following problem:
Write the following number in ordinary decimal form:
3.49 × 10−11
Solve the following problem:
Write the following number in ordinary decimal form:
5.16 × 104
What are the favourable qualities given to gold when it is alloyed with copper or silver for the purpose of making ornaments?
Give an example of each mixture having the following characteristics. Suggest a suitable method to separate the components of this mixture
Two immiscible liquids.
When light is passed through water containing a few drops of milk, it shows a bluish tinge. This is due to the ______ of light by milk and the phenomenon is called ______. This indicates that milk is a ______ solution.
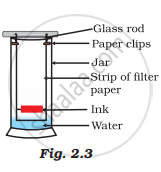
A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in Fig.2.3. The filter paper was removed when the water moved near the top of the filter paper.
(i) What would you expect to see, if the ink contains three different coloured components?
(ii) Name the technique used by the child.
(iii) Suggest one more application of this technique.
Match the following physical quantities with units
| Physical quantity | Unit |
| (i) Molarity | (a) g mL–1 |
| (ii) Mole fraction | (b) mol |
| (iii) Mole | (c) Pascal |
| (iv) Molality | (d) Unitless |
| (v) Pressure | (e) mol L–1 |
| (vi) Luminous intensity | (f) Candela |
| (vii) Density | (g) mol kg–1 |
| (viii) Mass | (h) Nm–1 |
| (i) kg |
