Advertisements
Advertisements
Question
What is photoelectri effect ? Defin (i) Stopping potential (ii) Photoelectric work function.
Solution
The phoenomenon of emission of electron by certain substance (metal), when it is exposed to radiation of suitable frequencies is called photo electric effect. Thus in photoelectric effect emitted electrons are called
photoelectrons.
(i) Stopping -potential : The minimum negative potential given to the plate for which photo electric current stop or become zero.
(ii) Photo electric work function : The minimum energy required to remove electron from a given surface is called photo electric work function
APPEARS IN
RELATED QUESTIONS
The photoelectric work function for a metal surface is 2.3 eV. If the light of wavelength 6800A is incident on the surface of metal, find threshold frequency and incident frequency. Will there be an emission of photoelectrons or not?
[Velocity of light c = 3 x 108 m/s,
Planck’s constant, h = 6.63 * 10-34 Js ]
The photoelectric current in a photoelectric cell can be reduced to zero by a stopping potential of 1.8 volt. Monochromatic light of wavelength 2200Å is incident on the cathode. Find the maximum kinetic energy of the photoelectrons in joules. [Charge on electron = 1.6 x 10-19 C]
Draw a neat labelled circuit diagram of experimental arrangement for study of photoelectric effect.
Write three characteristic features in photoelectric effect that cannot be explained on the basis of wave theory of light, but can be explained only using Einstein's equation.
Sketch the graphs showing variation of stopping potential with frequency of incident radiations for two photosensitive materials A and B having threshold frequencies vA > vB.
(i) In which case is the stopping potential more and why?
(ii) Does the slope of the graph depend on the nature of the material used? Explain.
The photoelectric work function for a metal is 4.2 eV. If the stopping potential is 3V, find the threshold wavelength and maximum kinetic energy of emitted electrons.
(Velocity of light in air = 3 x 108m/s,
Planck's constant = 6·63 x10-34 J -s,
Charg.e ori electron = 1·6 x 10 -19 C)
Light of intensity ‘I’ and frequency ‘v’ is incident on a photosensitive surface and causes photoelectric emission. What will be the effect on anode current when (i) the intensity of light is gradually increased. In each case, all other factors remain the same. Explain, giving justification in each case.
Light of intensity ‘I’ and frequency ‘v’ is incident on a photosensitive surface and causes photoelectric emission. What will be the effect on anode current when (ii) the frequency of incident radiation is increased. In each case, all other factors remain the same. Explain, giving justification in each case.
Light of intensity ‘I’ and frequency ‘v’ is incident on a photosensitive surface and causes photoelectric emission. What will be the effect on anode current when the anode potential is increased? In each case, all other factors remain the same. Explain, giving justification in each case.
Two monochromatic beams, one red and the other blue, have the same intensity. In which case (i) the number of photons per unit area per second is larger, (ii) the maximum kinetic energy of the photoelectrons is more? Justify your answer.
Draw a plot showing the variation of photoelectric current versus the intensity of incident radiation on a given photosensitive surface.
If the total energy of radiation of frequency 1014 Hz is 6.63 J, calculate the number of photons in the radiation. (Planck’s constant = 6.63 x 10–34 J.s.)
In an experiment of the photoelectric effect, the graph of maximum kinetic energy EK of the emitted photoelectrons versus the frequency v of the incident light is a straight line AB shown in Figure 6 below:
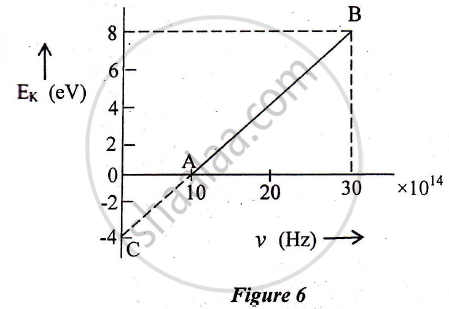
Find:
1) Threshold frequency of the metal
2) The work function of the metal.
3) Stopping potential for the photoelectrons emitted by the light of frequency `v = 30 xx 10^14 Hz`
A photosensitive surface emits photoelectrons when red light falls on it. Will the surface emit photoelectrons when blue light is incident on it? Give reason.
Use Einstein's photoelectric equation to explain the observations from this graph ?
What change will you observe if intensity of incident radiation is changed but the frequency remains the same?
A beam of monochromatic radiation is incident on a photosensitive surface. Answer the following question giving reason :
Do the emitted photoelectrons have the same kinetic energy?
With reference to the photoelectric effect, what is meant by threshold wavelength?
Two metals A and B have work functions 4 eV and 6 eV respectively. Which metal has a lower threshold wavelength for photoelectric effect?
Photoelectric effect is possible ______.
Light of wavelength 4000 Å is incident on two metals A and B. Which metal will emit photoelectrons, if their work functions are 3.8 e V and 1.6 e V respectively?
The phenomenon of photoelectric emission was observed by ______.
Consider an electron in front of metallic surface at a distance d (treated as an infinite plane surface). Assume the force of attraction by the plate is given as `1/4 q^2/(4πε_0d^2)`. Calculate work in taking the charge to an infinite distance from the plate. Taking d = 0.1 nm, find the work done in electron volts. [Such a force law is not valid for d < 0.1nm].
