Advertisements
Advertisements
Question
Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
Options
Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane
Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane
1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Solution
1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Explanation:
The attractions get stronger as the molecules get bigger in size and have more electrons.
APPEARS IN
RELATED QUESTIONS
Give reasons : n-Butyl bromide has higher boiling point than t-butyl bromide.
For the same alkyl group, an alkyl bromide has a higher boiling point than alkyl fluoride because:
Which of the following compounds has the highest boiling point?
Which of the following possesses the highest melting point?
Mg reacts with RBr best in ____________.
Arrange the following compounds in the increasing order of their densities.
(a)
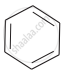
(b)
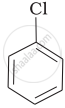
(c)
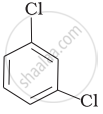
(d)
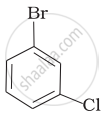
Assertion: The boiling points of alkyl halides decrease in the order:
\[\ce{RI > RBr > RCl > RF}\]
Reason: The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following organic halogen compound:
4-tert-Butyl-3-iodoheptane
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halide:
\[\ce{CH3 C(C2H5)2CH2Br}\]
