Advertisements
Advertisements
Question
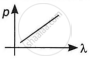
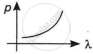
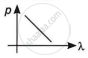
Which of the following graphs correctly represents the variation of a particle momentum with its associated de-Broglie wavelength?
Options
Solution

Explanation:
de-Broglie wavelength is the wavelength that is connected to an object in relation to its momentum and mass. Typically, the force of a particle is inversely proportional to its de-Broglie wavelength.
A photon's momentum is determined by:
P = `E/c = h/lambda` where E is the energy of the photon, c is the speed of light in vacuum, h is Planck's constant and λ is the de-Broglie wavelength.
According to de-Broglie, `p = h/lambda` or `p ∝ 1/lambda`.
By this relation, we can conclude that the linear momentum of a photon is inversely proportional to the de-Broglie wavelength. The graph of p vs λ shall be a rectangular hyperbola.
RELATED QUESTIONS
A proton and an α-particle have the same de-Broglie wavelength Determine the ratio of their speeds.
What is the
(a) momentum,
(b) speed, and
(c) de Broglie wavelength of an electron with kinetic energy of 120 eV.
What is the de Broglie wavelength of a bullet of mass 0.040 kg travelling at the speed of 1.0 km/s?
An electron and a photon each have a wavelength of 1.00 nm. Find
(a) their momenta,
(b) the energy of the photon, and
(c) the kinetic energy of electron.
For what kinetic energy of a neutron will the associated de Broglie wavelength be 1.40 × 10−10 m?
Show that the wavelength of electromagnetic radiation is equal to the de Broglie wavelength of its quantum (photon).
Describe briefly how the Davisson-Germer experiment demonstrated the wave nature of electrons.
Two particles A1 sand A2 of masses m1, m2 (m1 > m2) have the same de Broglie wavelength. Then ______.
- their momenta are the same.
- their energies are the same.
- energy of A1 is less than the energy of A2.
- energy of A1 is more than the energy of A2.
Given below are two statements:
Statement - I: Two photons having equal linear momenta have equal wavelengths.
Statement - II: If the wavelength of photon is decreased, then the momentum and energy of a photon will also decrease.
In the light of the above statements, choose the correct answer from the options given below.
A particle of mass 4M at rest disintegrates into two particles of mass M and 3M respectively having non zero velocities. The ratio of de-Broglie wavelength of particle of mass M to that of mass 3M will be: